solution
advertisement

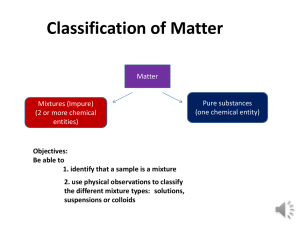
p. 1 Matter Guided Notes Student’s name:___________________________ Use with Matter Study Guide (ppt) 1. Matter is ________ that has a ________ and a ________ • examples:_______________ and ______________ • non-examples: _______________ and ______________ 2. States of matter: Rank the four states of matter from least orderly to most orderly: ______________________, ______________________, ______________________, ______________________ 3. Physical Change is changing from ______________________ to another. 4. Physical change can also be change in ______________________. 5. Chemical Change is the making of a whole new ____________________. 6. Physical change example with wood: ______________________________________________ __________________________ 7. Chemical change example with wood: ______________________________________________ _________________________________ 8. After a ______________________________, the old substance does not exist. 9. Riddle: Why doesn’t Atom have a pencil? 10.Answer: He only has a PEN (Protons, Electrons, and Neutrons). 11. Why is a molecule of water not an element? ______________________________________________ 12.Why is a molecule of nitrogen an element? ______________________________________________ 13.What is a molecule of water? _____________________________________________ period______ 14.Review from an earlier slide—What is hydrogen peroxide? ____________________________________ 15.A solution is molecules that are mixed up __________________, also called _______________________________. 16.Riddle: When is a mixture of sugar and water not able to be homogeneous? ________________________________________ 17.Make a Venn diagram with just three words: solution, solute, solvent. My diagram: 18.In drawing #1 of the “Making a Solution” slide, what color is the solute? 19.In drawing 2 of the “Making a Solution” slide, what color is the solvent? 20.Bonus: In drawing #3 of the “Making a Solution” slide, what is inaccurate about the drawing? Answer: In an actual solution, you would no longer be able to __________________ the solute. 21.What are the two basic types of mixtures? _______________________ and ________________________ 22.What are the two types of homogeneous mixtures? ___________________ and ________________________ 23.At a glance, how could you tell a difference between the two types of homogeneous mixtures? ____________________________________________ 24.What is one type of heterogeneous mixture?_____________________ 25.What is one example of a heterogeneous mixture? _________________ p. 2 Matter Guided Notes Student’s name:___________________________ 26.Use the word bank to complete the graphic organizer. period______