Bonding and Intramolecular Forces
advertisement

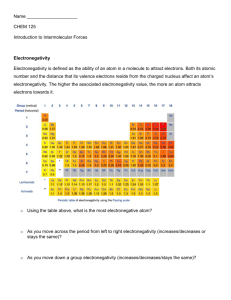
Intramolecular Forces – Types of Bonding Electronegativity is a measure of the ability of an atom or molecule to attract electrons in a chemical bond. The type of bond between two atoms is related to the difference in electronegativity (∆EN) of the two atoms that are bonded together: ∆EN = EN2 – EN1 , where EN2 is the element with the higher electronegativity EN1 is the element with the lower electronegativity The electronegativity of all the elements can be found on the Electronegativity Periodic Table. There is a clear periodic trend to electronegativity values – it increase up a group and across a period. Let’s look at an example: ammonia (NH3). The nitrogen atom has an electronegativity value of 3.04, while hydrogen has an electronegativity of 2.20. Therefore, the bonded pair of electrons in any N-H bond has a ∆EN = 0.84. The greater the ∆EN value, the greater the attraction of an electron pair to the atom of higher electronegativity (EN2). Although bonding is considered a continuum from pure covalent character (∆EN = 0) to increasing ionic character, certain ∆EN ranges correspond to one of the three general types of bonds. See the table below for reference: ∆EN 0.0 to 0.3 0.3 to 1.7 1.7 to 3.3 Type Covalent (non-polar) Polar Covalent (polar) Ionic (very polar) NON-POLAR 0.0 POLAR 0.3 Description Equal sharing of electrons Unequal sharing of electrons Transfer of electrons IONIC 1.7 3.3 For our example of ammonia (NH3), ∆EN = ENN – ENH = 3.04 – 2.20 = 0.84. This bond is therefore classified as a polar covalent bond. Bond Polarity In ammonia, the two atoms (nitrogen and hydrogen) have different electronegativities (i.e., ∆EN > 0). Just like any other polar covalent bond, the two bonding electrons are not equally shared between the two atoms (nitrogen and hydrogen). Because of this, a bond dipole exists. The atom that has a greater attraction for electrons (more electronegative) has a partial negative charge (-), and the atom that has a lower electronegativity has a partial positive charge (+). For ammonia, this can be drawn like the following: Molecular Polarity The composition and arrangement of the atoms in a molecular structure dictates the shift of the electron density, and thus, determines the polarity of the molecule. The absence of dipole moments renders the molecule non-polar. For example, diatomic molecules (i.e., H2, O2, F2, Br2, I2, N2, Cl2) contain two atoms of the same element; they do not have dipole moments. Binary molecules - molecules containing atoms of different elements – are polar molecules because they have a dipole moment. The following structures show the partial charges and the shift in the electron density for hydrogen chloride: However, it is important to note that the presence of polar bonds in a molecule does not guarantee that the molecule itself will be polar. To determine the polarity of a molecule, you must find the vector sum of all bond dipoles. If the vectors cancel out, the molecule is nonpolar. If the vectors do not cancel out, the molecule is polar. The following examples are non-polar molecules. For each vector, there is a vector of equal proportion that is pulling in an opposite direction. Therefore, the vectors all cancel out. The following examples are polar molecules. Unlike the previous examples, not all vectors cancel out. Notice that both polar molecules and non-polar molecules can have lone electron pairs. ______________________________________________________________________________ Intermolecular Forces of Attraction The four main types of attractive forces that exist between neighbouring particles are: 1. 2. 3. 4. London dispersion forces Dipole-dipole forces Hydrogen bonding Ion-dipole forces These attractions between particles determine the physical properties of substances, which includes the boiling point, freezing point, vapour pressure, and compressibility. London Dispersion Forces - London dispersion forces of attraction exist between all particles They are the weakest force of attraction Are due to the instantaneous dipole-induce dipole attraction London dispersion forces polarize electrons The only force of attraction between non-polar molecules; noble gases also exhibit London forces of attraction The constant motion of the electrons and their “clouds” will produce momentary dipoles in the molecule and any neighbouring molecules’ electrons will shift away because of the repulsion between like charges. The momentary force of attraction is the London dispersion force. Diagram of how London Dispersion Forces induce dipoles on neighbouring atoms: Dipole-Dipole Forces - Exist between polar molecules Stronger than London dispersion forces An attraction between the negative dipole of one molecule to the positive dipole of another molecule The force of attraction increases significantly between molecules as the distance decreases Hydrogen Bonding - Hydrogen bonding is a special case of dipole-dipole attractions Occurs between molecules in which hydrogen is covalently bonded to a small, very electronegative atom, principally nitrogen, oxygen or fluorine Ion-Dipole Forces - An ion is attracted to an oppositely charged dipole of a polar molecule The size and charge of the ion, as well as the magnitude of the dipole moment of the polar molecule are factors in the strength of the ion-dipole force Bonding and Molecular Shape We’ll do these examples together so that everything makes sense! Example 1: Chemical bonds are indicated below for some gaseous substances. Indicate which atom will be more positive and which atom will be more negative in each bond. If both atoms have equal positive or negative character, state that the bond is non-polar. Na – Cl C–F P–O C–C K–H H H I I H–C–C–H I I H H Example 2: Arrange the following molecules in decreasing order of polarity. F–F C–F H–F C–H Na – F Example 3: Classify the bonds in each of the following substances as covalent, polar covalent, or ionic: K2 O KCl BeO CBr4 KH N2 SiF4 Lewis Structures, Molecular Shape, and Molecular Dipoles 1. For each of the following molecules: i) Draw the Lewis structure and the molecular shape. ii) Find ∆EN. iii) Indicate the direction of any bond dipoles with vectors (arrows). iv) Add the dipoles and determine if a molecular dipole exists. a) LiF b) CH4 c) HCl d) Cl2 e) NH3 f) N2F4 g) BCl3 h) BeH2 i) H2Se j) BF2H h) SFCl5 i) CH2F2 2. Which of the following molecules has a molecular dipole? a) NaF (g) b) MgO (g) c) MgI2 (g) e) CO2 (g) f) H2S (g) g) CCl4 (g) d) BCl3 (g) h) PF3 (g)



![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)