Group Dipole Worksheet
advertisement
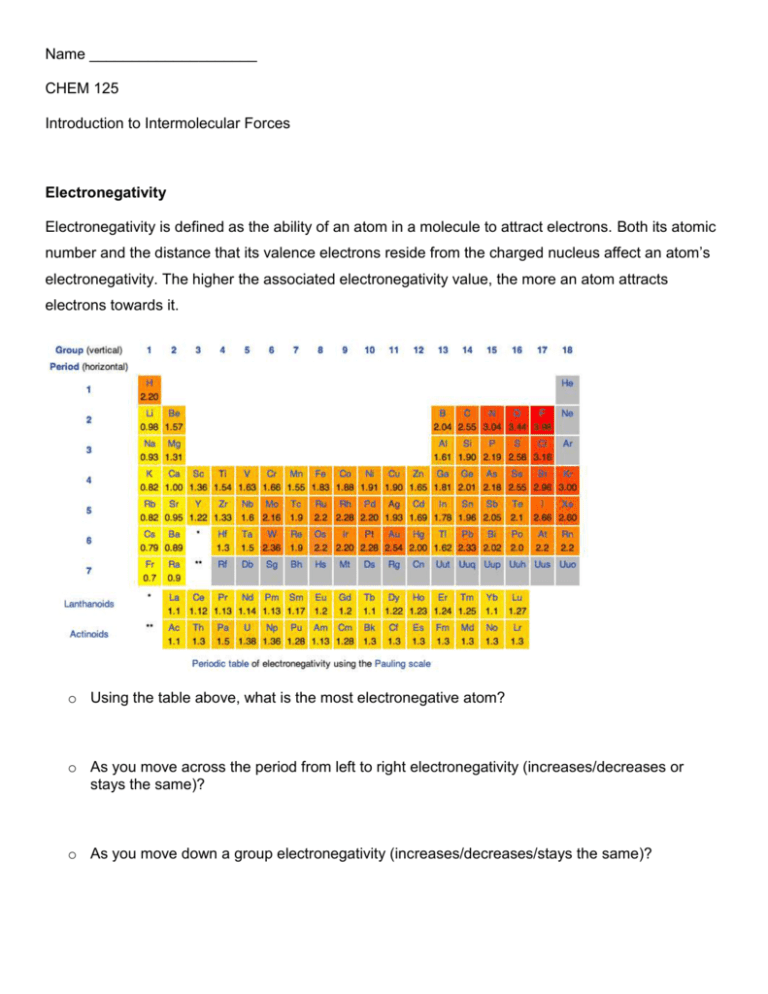
Name ____________________ CHEM 125 Introduction to Intermolecular Forces Electronegativity Electronegativity is defined as the ability of an atom in a molecule to attract electrons. Both its atomic number and the distance that its valence electrons reside from the charged nucleus affect an atom’s electronegativity. The higher the associated electronegativity value, the more an atom attracts electrons towards it. o Using the table above, what is the most electronegative atom? o As you move across the period from left to right electronegativity (increases/decreases or stays the same)? o As you move down a group electronegativity (increases/decreases/stays the same)? Bond Dipoles and Dipole Moments Polar covalent bonds form between atoms of different electronegativity (can not be same atom). In other words, when the electronegativity difference between the bonding atoms is sufficient to cause partial charges on the bonding atoms (but not so large as to produce an ionic bond), that is considered a polar covalent bond. NF3 (shown below) has polar covalent bonds. Fluorine is more electronegative than nitrogen; therefore the fluorine atom pulls the electrons from the nitrogen. Thus the fluorine atoms are slightly electron-rich (-) and the nitrogen atom is slightly electron poor (+). This is described as a bond dipole and is represented using an arrow with a cross at one end to indicate the direction of electron displacement. ( ) o Draw in the partial charges [(-) and (+)] on both NF3 and NH3. o Draw the molecule H2O, with its correct geometry, and show the bond dipoles and partial charges. Just as individual bonds in a molecule are often polar, molecules as a whole are also often polar because of the net sum of individual bond polarities. The resultant molecular dipoles (dipole moment or ) can be looked at in the following way: Assume that there is a “center of mass” of all positive charges (nuclei) in a molecule and a “center of mass” of all negative charges (electrons). If these two centers don’t coincide, then the molecule has a net polarity. In contrast, carbon dioxide and carbon tetrabromide (CBr4) have zero dipole moments. Molecules of both substances contain individual polar covalent bonds, but because of the symmetry (remember molecular geometry) of their structures, the individual bond polarities exactly cancel. o Draw both CBr4 and CO2 with the correct molecular geometry. o Draw in the dipoles on both CBr4 and CO2. o Explain why these two compounds have = 0. o Complete the following table to decide if the molecular contains polar bonds and/or if the molecule itself is polar. o Complete the following table to decide if the molecular contains polar bonds and/or if the molecule itself is polar.





![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)





