APS B Chapter 4 Preparation Explain the process for determining
advertisement

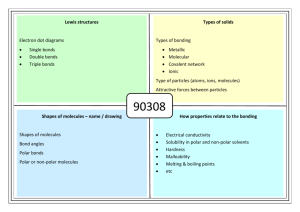
APS B Chapter 4 Preparation 1. Explain the process for determining the polarity of bonds in molecules. 2. Order the following bonds from lowest to highest polarity. a. C-O c. C-Br e. Li-Cl b. N-O d. U-O f. F-F 3. Explain how the behavior of electrons in metallic bonds accounts for the ability of metals to transfer electricity and heat. 4. Write the delta and arrow notations for the polarity of the following molecules. If they are non-polar, write “non-polar.” a. Br2O c. F2 b. LiCl d. MgCl2 5. Why do polar molecules tend to have higher boiling points than non-polar ones? 6. How do increasing numbers of electron shells make a molecule less electronegative? 7. Identify the kinds of bonding (ionic, metallic, polar covalent, non-polar covalent) taking place in these molecules, and explain how you know. a. Na2 c. HF e. F2 b. LiCl d. CuO2 f. NO2 8. Explain why oil and water do not mix (and which molecules are responsible for this separation.) 9. Explain the process for determining the polarity of molecules. 10. Create Lewis dot diagrams for the following molecules. a. H2S c. CO2 e. KCN b. NH3 d. H2O f. CH2Br2 11. Explain how an HCl molecule can induce a dipole in a Cl2 molecule. 12. Does symmetry tend to increase or decrease the polarity of a molecule? Explain. 13. BaCl2 is an ionic compound. Describe what will happen to it if it is placed in a polar liquid (like water.) 14. Draw two water molecules oriented so that a hydrogen bond would be between them, and indicate it with a dashed line.