The Ionosphere
advertisement

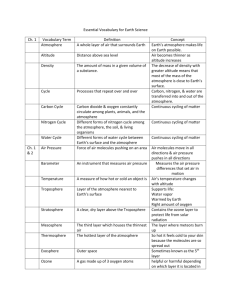
Standard 6.6 The student will investigate and understand the properties of air and the structure and dynamics of the Earth’s atmosphere. concepts include air as a mixture of gaseous elements and compounds; air pressure, temperature, and humidity; how the atmosphere changes with altitude; natural and human-caused changes to the atmosphere; the relationship of atmospheric measures and weather conditions; basic information from weather maps including fronts, systems, and basic measurements; and the importance of protecting and maintaining air quality. Essential Knowledge, Skills, and Processes In order to meet this standard, it is expected that students should be able to: comprehend and apply basic terminology related to air and the atmosphere. identify the composition and physical characteristics of the atmosphere. analyze and interpret charts and graphs of the atmosphere in terms of temperature and pressure. measure and record air temperature, air pressure, and humidity in using appropriate units of measurement and tools. analyze and explain some of the effects that natural events and human activities may have on weather, atmosphere, and climate. map the movement of cold and warm fronts, and interpret their effects on observable weather conditions. design an investigation to relate temperature, barometric pressure, and humidity to changing weather conditions. interpret basic weather maps, and make forecasts based on the information presented. compare and contrast cloud types, and relate cloud types to weather conditions. compare and contrast types of precipitation. compare and contrast weather-related phenomena including thunderstorms, tornados, hurricanes, and drought. evaluate their own roles in protecting air quality. Earth’s Atmoshere is a blanket of gases. At the surface, there is a balance between destruction (output) and production (input) of these gases. For example, nitrogen is removed from the atmosphere primarily by biological processes that involve soil bacteria. It is returned to the atmosphere mainly through the decaying of plant and animal matter. Oxygen, on the other hand, is removed from the atmosphere when organic matter decays and when oxygen combines with other substances, producing oxides. It is also taken from the atmosphere during breathing, as the lungs take in oxygen and release carbon dioxide (CO2). The addition of oxygen to the atmosphere occurs during photosynthesis, as plants, in the presence of sunlight, combine carbon dioxide and water to produce sugar and oxygen. The concentration of the invisible gas water vapor (H20), however, varies greatly from place to place, and from time to time. Close to the surface in warm, steamy, tropical locations, water vapor may account for up to 4 percent of the atmospheric gases, whereas in colder arctic areas, its concentration may dwindle to a mere fraction of a percent (see Table TABLE 1.1 The earth's atmosphere as viewed from space. The thin blue area near the horizon shows the shallowness of the earth's atmosphere. 1.1). Water vapor molecules are, of course, invisible. They become visible only when they transform into larger liquid or solid particles, such as cloud droplets and ice crystals. The changing of water vapor into liquid water is called condensation, whereas the process of liquid water becoming water vapor is called evaporation. In the lower atmosphere, water is everywhere. It is the only substance that exists as a gas, a liquid, and a solid at those temperatures and pressures normally found near the earth's surface (see Fig. 1.3). Water vapor is an extremely important gas in our atmosphere. Not only does it form into both liquid and solid cloud particles that grow in size and fall to earth as precipitation, but it also releases large amounts of heat—called latent heat— when it changes from vapor into liquid water or ice. Latent heat is an important source of atmospheric energy, especially for storms, such as thunderstorms and hurricanes. Moreover, water vapor is a potent greenhouse gas because it strongly absorbs a portion of the earth's outgoing radiant energy (somewhat like the glass of a greenhouse prevents the heat Composition of the Atmosphere Near the Earth's Surface VARIABLE GASES PERMANENT GASES s - Percent Gas Symbol (by Volume) Dry Air Percent Parts per Gas (and Particles) Symbol (by Volume) Nitrogen Oxygen Argon Neon Helium Hydrogen Xenon N2 O2 Ar Ne He H2 Xe 78.08 20.95 0.93 0.0018 0.0005 0.00006 0.000009 Water vapor Carbon dioxide Methane Nitrous oxide Ozone Particles (dust, soot, etc.) Chlorofluorocarbons (CFCs) H2O CO2 CH4 N2O O3 0.000001 0.00000002 0 to 4 0.036 0.00017 0.00003 0.000004 0.01-0.15 0.0002 *For CO,, 365 parts per million means that out of every million air molecules, 365 are CO, molecules. "Stratospheric values are about 5 to 12 ppm. Million (ppm)* 365* 1.7 0.3 0.04** http://www.srh.noaa.gov/jetstream/atmos/layers.htm very nice general site for info http://www.youtube.com/watch?v=3CerJbZ-dm0&safety_mode=true&persist_safet y_mode=1&safe=active nice Irish accent 7min. jet flight up through layers of atmosphere. Kittinger’s high altitude parahute http://www.dailymail.co.uk/news/article-2279020/Russian-meteorite-Moment-meteorite-exploded-doctors-treat-500-people-injured. html meteor blows up in Russia 2/14/13 http://online.wsj.com/article/SB10001424127887323864604579069730686941454.html?mod=WSJ_hpp_MIDDLENexttoWhatsNe wsForth Google’s fleet of jets 2.3 million gallons a year The layers of the atmosphere are determined based on TEMPERATURE TRENDS Rise throught the troposphere…..temps decrease Rise through the stratosphere…..temps increase Rise through the mesosphere…..temps decrease And rise through the thermosphere…..temps increase(although you wouldn’t feel it) As you rise through the Troposphere, the temp falls. This temp drop is about 6.5 degree C. drop for every 1000 m.(1km). That is about 40 F. per 1000 feet. Somewhere around 12 km (7mi) up the temp trends higher so we have left the Troposphere and entered the next layer, Stratosphere. In the Troposphere we find all of our weather. Weather is caused by the UNEVEN HEATING of the Earth’s surface. Also largely involved is the water cycle. Evaporation, Transpiration, Condensation, Precipitation ATMOSPHERE TROPOSPHERE -from Greek tropos means “turning” or “mixing” -lowest portion of Earth’s atomosphere….zero to 10 km up At the equator it is around 11-12 miles (18-20 km) high, at 50°N and 50°S, 5½ miles and at the poles just under four miles high. -contains approximately 75% of atmosphere’s mass -averages about 11 km thick (depth) /starts at Earth and goes up to around 7 miles -closest layer is influenced with friction of Earth’s surface changing air flow -all of our weather is here….clouds, storms, hurricanes, etc. -chemical composition mostly uniform ….except for water vapor -temperature decreases with height -water vapor decreases with temp drop so water vapor decreases with height STRATOSPHERE -second layer of Earth’s atmosphere ….10km to 50 km /up to around 31 miles -stratified by layers….colder lower going warmer as you climb higher -top level of this layer has a temp of approx. –3oC (just below freezing) -temp heated by UV radiation and increases with height -jets climbs to here to use the Jet Stream advantages -the ozone layer is found here. Protects us from harmful UV radiation. -weather balloons can reach this layer Stratosphere holds around 24% of all atmospheric mass Troposphere plus Stratosphere combine for 99% of atmosphere’s mass MESOSPHERE -from Greek mesos=middle, and sphaira=ball -50 km to about 80-90 km above earth -temperature decreases with altitude in this layer -this layer is between maximum altitude for aircraft and minimum altitude for spacecraft -most poorly understood part of our atmosphere -meteors burn up here due to increasing molecules closer to Earth THERMOSPHERE -from Greek word for heat -largest layer of our atmosphere, above mesosphere and below exosphere -starts about 90 km above earth and up to about 500-1000 km above earth (or outer space) -temperatures rise with altitude here rising to as much as 1500 C. -you would not feel this heat as heat is transferred between particles and there are extremely few particles in the Thermosphere. A normal thermometer would read well below 0 C. due to the near vacuum of particles found here. -temperature is highly dependent on solar activity at this point -radiation here causes atmospheric particles here to become electrically charged (ionosphere) allowing radio waves to “bounce off” this layer http://www.youtube.com/watch?v=AV5T-40EG0U&list=PLF260DAA31E5ED448 short, simple explaination of layers -space shuttle and International Space Station orbit here http://www.windows2universe.org/earth/Atmosphere/thermosphere.html Temperatures climb sharply in the lower thermosphere (below 200 to 300 km altitude), then level off and hold fairly steady with increasing altitude above that height. Solar activity strongly influences temperature in the thermosphere. The thermosphere is typically about 200° C (360° F) hotter in the daytime than at night, and roughly 500° C (900° F) hotter when the Sun is very active than at other times. Temperatures in the upper thermosphere can range from about 500° C (932° F) to 2,000° C (3,632° F) or higher. Finally, the aurora (the Southern and Northern Lights) primarily occur in the thermosphere. Charged particles (electrons, protons, and other ions) from space collide with atoms and molecules in the thermosphere at high latitudes, exciting them into higher energy states. Those atoms and molecules shed this excess energy by emitting photons of light, which we see as colorful auroral displays. The Ionosphere Scientists call the ionosphere an extension of the thermosphere. So technically, the ionosphere is not another atmospheric layer. The ionosphere represents less than 0.1% of the total mass of the Earth's atmosphere. Even though it is such a small part, it is extremely important! The upper atmosphere is ionized by solar radiation. That means the Sun's energy is so strong at this level, that it breaks apart molecules. So there ends up being electrons floating around and molecules which have lost or gained electrons. When the Sun is active, more and more ionization happens! Different regions of the ionosphere make long distance radio communication possible by reflecting the radio waves back to Earth. http://www.srh.noaa.gov/jetstream/atmos/ionosphere_max.htm The Earth’s ionosphere and ground form a “waveguide” through which VLF radio signals can propagate or “bounce” around the Earth. Image courtesy Morris Cohen, Stanford University EXOSPHERE -often considered part of the thermosphere -the outermost layer of earth’s atmosphere. Very, very few particles are in this layer -particles here can actually reach “escape velocity” and leave this layer’s weak gravitational pull. -this layer begins around 500-1000 km up and tails off into outer space -repeat; there are almost no atoms or particles here. The lightest atoms are found here such as hydrogen, helium, carbon dioxide and atomic oxygen. Aurora Borealis An aurora (plural: auroras or aurorae) is a natural light display in the sky particularly in the high latitude (Arctic and Antarctic) regions, caused by the collision of energetic charged particles with atoms in the high altitude atmosphere (thermosphere). The charged particles originate in the magnetosphere and solar wind and, on Earth, are directed by the Earth's magnetic field into the atmosphere. Aurora is classified as diffuse or discrete aurora. Its southern counterpart, the aurora australis (or the southern lights), has almost identical features to the aurora borealis and changes simultaneously with changes in the northern auroral zone and is visible from high southern latitudes in Antarctica, South America and Australia. What Causes Them? Auroras are result from emissions of photons in the Earth's upper atmosphere, above 80 km (50 mi), from ionized nitrogen atoms regaining an electron, and oxygen and nitrogen atoms returning from an excited state to ground state. They are ionized or excited by the collision of solar wind and magnetospheric particles being funneled down and accelerated along the Earth's magnetic field lines; excitation energy is lost by the emission of a photon of light, or by collision with another atom or molecule: Oxygen emissions cause a green or brownish-red, depending on the amount of energy absorbed. Nitrogen emissions cause bluelue or red. Blue if the atom regains an electron after it has been ionized. Red if returning to ground state from an excited state. Sweden http://www.gi.alaska.edu/AuroraForecast Interesting site for aurora info and schedules http://roble.pntic.mec.es/rmac0040/watercycle.html http://kilby.sac.on.ca/faculty/dgalajda/enviro/atmosphere.htm http://www.srh.noaa.gov/jetstream/atmos/atmprofile.htm http://hyperphysics.phy-astr.gsu.edu/hbase/pman.html#bar (barometric pressure illustration/explain http://www.kidsgeo.com/geography-for-kids/0048-temperature-effects-on-atmosphere.php (temp chart) UV radiation Simply put, ultraviolet radiation (also known as UV radiation or ultraviolet rays) is a form of energy traveling through space. Office of Air and Radiation (6205J); June 2010; EPA 430-F-10-025 The sun emits energy over a broad spectrum of wavelengths: visible light that you see, infrared radiation that you feel as heat, and ultraviolet (UV) radiation that you can’t see or feel. UV radiation has a shorter wavelength and higher energy than visible light. It affects human health both positively and negatively. Short exposure to UVB radiation generates vitamin D, but can also lead to sunburn depending on an individual’s skin type. Fortunately for life on Earth, our atmosphere’s stratospheric ozone layer shields us from most UV radiation. What does get through the ozone layer, however, can cause the following problems, particularly for people who spend unprotected time outdoors: Skin cancer Cataracts Suppression of the immune system Premature aging of the skin Since the benefits of sunlight cannot be separated from its damaging effects, it is important to understand the risks of overexposure, and take simple precautions to protect yourself. Did You Know? Ultraviolet (UV) radiation, from the sun and from tanning beds, is classified as a human carcinogen, according to the U.S. Department of Health and Human Services and the World Health Organization. Types of UV Radiation The stratospheric ozone layer screens out much of the sun’s harmful UV rays. Scientists classify UV radiation into three types or bands—UVA, UVB, and UVC. The ozone layer absorbs some, but not all, of these types of UV radiation: UVA: Wavelength: 320-400 nm. Not absorbed by the ozone layer. UVB: Wavelength: 290-320 nm. Mostly absorbed by the ozone layer, but some does reach the Earth’s surface. UVC: Wavelength: 100-290 nm. Completely absorbed by the ozone layer and atmosphere. UVA and UVB radiation that reaches the Earth’s surface contributes to the serious health effects listed above; it also contributes to environmental impacts. Levels of UVA radiation are more constant than UVB, reaching the Earth’s surface without variations due to the time of day or year. In addition, UVA radiation is not filtered by glass. UV Levels Depend on a Number of Factors The level of UV radiation reaching the Earth’s surface can vary. Each of the following factors can increase your risk of UV radiation overexposure and consequent health effects. Stratospheric Ozone Layer The amount of UV rays the ozone layer absorbs varies depending on the time of year and other natural events. Additionally, the ozone layer is thinner than it used to be due to ozone-depleting chemicals used in industry and consumer products. These chemicals are being phased out, but the ozone layer is not predicted to heal to pre-1980 levels until mid– to late-century. Time of Day The sun is highest in the sky around noon. At this time, the sun’s rays have the least distance to travel through the atmosphere and UVB levels are at their highest. In the early morning and late afternoon, the sun’s rays pass through the atmosphere at an angle and their intensity is greatly reduced. Time of Year The sun’s angle varies with the seasons, causing the intensity of UV rays to change. UV intensity tends to be highest in the summer. Latitude The sun’s rays are strongest at the equator, where the sun is most directly overhead and UV rays must travel the least distance through the atmosphere. Ozone also is naturally thinner in the tropics compared to the mid- and high-latitudes, so there is less ozone to absorb the UV radiation as it passes through the atmosphere. At higher latitudes, the sun is lower in the sky, so UV rays must travel a greater distance through ozone-rich portions of the atmosphere and, in turn, expose those latitudes to less UV radiation. Altitude UV intensity increases with altitude because there is less atmosphere to absorb the damaging rays. As a result, your chance of damaging your eyes and skin increases at higher altitudes. Weather Conditions Cloud cover reduces UV levels, but not completely. Depending on the thickness of the cloud cover, it is possible to burn on a cloudy day, even if it does not feel warm. Reflection Surfaces like snow, sand, pavement, and water reflect much of the UV radiation that reaches them. Because of this reflection, UV intensity can be deceptively high even in shaded areas. http://www.epa.gov/sunwise/doc/uvradiation.html DNA readily absorbs UV-B radiation, which commonly changes the shape of the molecule in one of several ways. The illustration below illustrates one such change in shape due to exposure to UV-B radiation. Changes in the DNA molecule often mean that protein-building enzymes cannot “read” the DNA code at that point on the molecule. As a result, distorted proteins can be made, or cells can die. Ultraviolet (UV) photons harm the DNA molecules of living organisms in different ways. In one common damage event, adjacent bases bond with each other, instead of across the “ladder.” This makes a bulge, and the distorted DNA molecule does not function properly. (Illustration by David Herring) But living cells are “smart.” Over millions of years of evolving in the presence of UV-B radiation, cells have developed the ability to repair DNA. A special enzyme arrives at the damage site, removes the damaged section of DNA, and replaces it with the proper components (based on information elsewhere on the DNA molecule). This makes DNA somewhat resilient to damage by UV-B. In addition to their own resiliency, living things and the cells they are made of are protected from excessive amounts of UV radiation by a chemical called ozone. A layer of ozone in the upper atmosphere absorbs UV radiation and prevents most of it from reaching the Earth. Yet since the mid-1970s, human activities have been changing the chemistry of the atmosphere in a way that reduces the amount of ozone in the stratosphere (the layer of atmosphere ranging from about 11 to 50 km in altitude). This means that more ultraviolet radiation can pass through the atmosphere to the Earth’s surface, particularly at the poles and nearby regions during certain times of the year. Without the layer of ozone in the stratosphere to protect us from excessive amounts of UV-B radiation, life as we know it would not exist. Scientific concern over ozone depletion in the upper atmosphere has prompted extensive efforts to assess the potential damage to life on Earth due to increased levels of UV-B radiation. Some effects have been studied, but much remains to be learned. AIR PRESSURE Air pressure decreases as one travels higher (away from Earth) in our atmosphere. Air pressure matters to us as it affects our body (we have always lived in it so we don’t realize how much it is pushing down on use). Differences in air pressure causes wind and condensation of water molecules (=precipitation or not) so it largely causes our weather. http://www.youtube.com/watch?v=9TKk7-uZw1c&feature=related&safety_mode=true &persist_safety_mode=1&safe=active Albuquerque Balloon Festival