reactions constants
advertisement
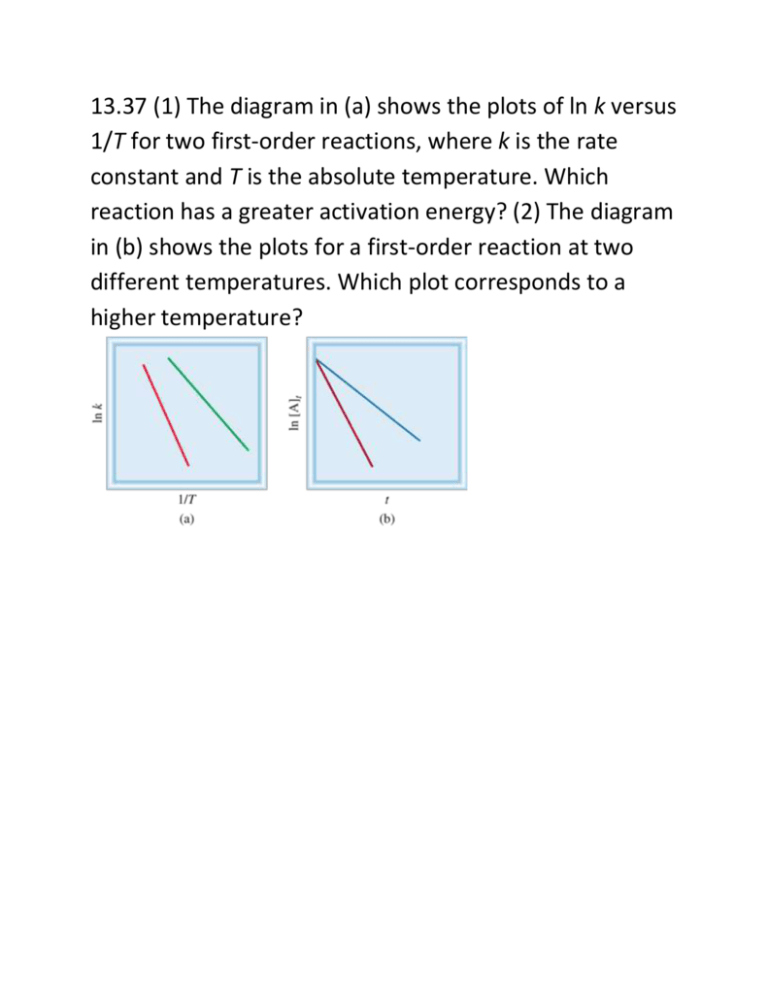
13.37 (1) The diagram in (a) shows the plots of ln k versus 1/T for two first-order reactions, where k is the rate constant and T is the absolute temperature. Which reaction has a greater activation energy? (2) The diagram in (b) shows the plots for a first-order reaction at two different temperatures. Which plot corresponds to a higher temperature? 13.38 Given the same reactant concentrations, the reaction at 250°C is 1.50 × 103 times as fast as the same reaction at 150°C. Calculate the activation energy for this reaction. Assume that the frequency factor is constant. 13.40 Variation of the rate constant with temperature for the first-order reaction is given in the following table. Determine graphically the activation energy for the reaction. 13.41 For the reaction the frequency factor A is 8.7 × 1012 s−1 and the activation energy is 63 kJ/mol. What is the rate constant for the reaction at 75°C? 13.42 The rate constant of a first-order reaction is 4.60 × 10−4 s−1 at 350°C. If the activation energy is 104 kJ/mol, calculate the temperature at which its rate constant is 8.80 × 10−4 s−1. 13.43 The rate constants of some reactions double with every 10-degree rise in temperature. Assume that a reaction takes place at 295 K and 305 K. What must the activation energy be for the rate constant to double as described? 13.44 Consider the first-order reaction Given that the frequency factor and activation energy for the reaction are 3.98 × 1013 s−1 and 161 kJ/mol, respectively, calculate the rate constant at 600°C. 13.45 Consider the second-order reaction Given that the frequency factor and activation energy for the reaction are 4.0 × 109/M · s and 85 kJ/mol, respectively, calculate the rate constant at 500°C. 13.46 The rate at which tree crickets chirp is 2.0 × 102 per minute at 27°C but only 39.6 per minute at 5°C. From these data, calculate the “activation energy” for the chirping process. (Hint: The ratio of rates is equal to the ratio of rate constants.)