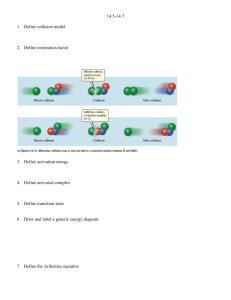
Chemical Kinetics Exercises: Sections 14.5: Temperature and Rate 1.
advertisement

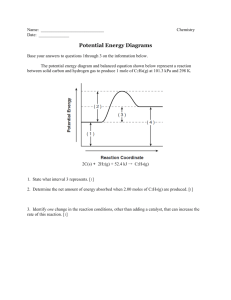
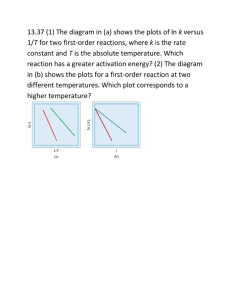
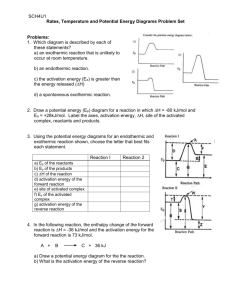
Name ______________________________________________ Date ____________________ Period ___ Homework Chapter 14: Chemical Kinetics Exercises: Sections 14.5: Temperature and Rate 1. (a) Explain the rate of a unimolecular (that is, one-molecule) reaction such as the isomerization of methyl isonitrile, Figure 14.15 on page 594 of the textbook, in terms of the collision model. (b) In a reaction of the form A(g) + B(g) → products, are all collisions of A with B that are sufficiently energetic likely to lead to reaction? Explain. (c) How does the kinetic molecular theory help us understand the temperature dependences of chemical reactions? 2. (a) Calculate the fraction of methyl isonitrile molecules that have an energy of 80.0 kJ or greater at 450 K. (b) Calculate the fraction for a temperature of 460 K. (c) What is the ratio of the fraction at 460 K to that at 450 K? 1 3. The gas-phase reaction, Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall enthalpy change of ─ 66 kJ. The activation energy for the reaction is 7 kJ. (a) Sketch the energy profile for the reaction, and label Ea and ΔE. (b) What is the activation energy for the reverse reaction? 4. Based on the following activation energies and energy changes: (Assume that all collision factors are the same) Reaction (a) (b) (c) Ea (kJ/ mol) 55 65 35 ΔE (kJ/ mol) – 45 – 10 10 (b) Which of the reactions will be the fastest in forward direction? The slowest? Explain your answer in each case. 2 4. Continued: (a) Draw an energy profile for each reaction; label Ea and ΔE on each profile. (c) Calculate the activation energy needed for each reaction in the reverse direction. Follow ALL math work rules! (d) Which of the reactions will be fastest in the reverse direction? The slowest? Explain your answer in each case. 5. Two similar reactions have the same rate constant at 25oC, but at 35oC one of the reactions has a higher rate constant than the other. Account for these observations. 3 6. A certain first-order reaction has a rate constant of 2.75 x 10 –2 s – 1 at 20oC. What is the value of k at 60oC if (a) Ea = 75.5 kJ/ mol Follow ALL math work rules! (b) Ea = 105 kJ/ mol Follow ALL math work rules! 7. The temperature dependence of the rate constant for the reaction CO(g) + NO2(g) → CO2(g) + NO(g) Is tabulated below. Temperature (K) k (M – 1 s 600 650 700 750 800 0.028 0.22 1.3 6.0 23 – 1) (a) Calculate Ea and A. Follow ALL math work rules! (A = the "frequency factor") 4