CH 117 Fall 2015Worksheet 3 The reaction 2 NOBr (g) ® 2 NO (g) +
advertisement

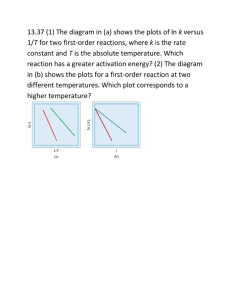
CH 117 Fall 2015 Worksheet 3 1. The reaction 2 NOBr (g) 2 NO (g) + Br2 (g) is a second order reaction with respect to NOBr with a k = 0.810 M-1s-1 at 10oC. If the initial concentration of NOBr is 7.5 x 10-3 M, how much NOBr will be left after a reaction time of 10 minutes? 2nd order, so use 2nd order integrated rate equation 1/[A]t = kt + 1/[A]o solving for [A]t 1/[A]t = (0.810 M-1s-1)(600 s) + 1/(7.5 x 10-3 M) = 619.333 [A]t = 0.0016 M 2. Define the activation energy of a reaction. The activation energy is the amount of energy necessary to get a reaction to progress or go forward. The “hump” you have to get over in terms of energy requirements. 3. What would happen to the rate constant of a reaction if the temperature of a reaction changes temperature from 350K to 100K? The rate constant of a reaction increases as the temperature increases and decreases as the temperature decreases. Since the temperature is decreasing from 350K to 100K, the rate constant value will also decrease. The decomposition of hydrogen peroxide at 25oC has a rate constant of 3.7 x 10-5 s-1 and at 55oC has a rate constant of 1.7 x 10-3 s-1. A) What is activation energy of this reaction in kJ/mol? B) What is the rate constant for this reaction at 35oC? This problem requires using a derivation of the Arrhenius equation: ln (k2/k1) = Ea/R (1/T1 – 1/T2) some pitfalls of using this equation include messing up parentheses with your calculator, and mixing up which rate constants correspond to which temperatures, so BE CAREFUL. The K2 and T2 rate constants and temperatures must go with each other and the K1 and T1 must go with each other. You will know to use this formula if you are given or are solving for activation energy or if you are given two different rate constants at two different temperature. To solve for the activation energy, plug and chug: temperature must be in Kelvin (convert Celsius to Kelvin by adding 273) and answer of the activation energy will come out in Joules! a) ln (1.7 x 10-3/3.7 x 10-5) = Ea/8.314 (1/298 – 1/328) 3.827 = Ea/8.314 (3.07 X 10-4) 12470.4 = Ea/8.314 103678.9 J/mol = Ea Ea = 103.7 kJ/mol b) To find the rate constant at a new temperature, use any one of the k/T combinations from the problem and factor in the activation energy you just solved for (same equation from above!): ln (k2/3.7 x 10-5) = 103700/8.314 (1/298 – 1/308) CH 117 Fall 2015 Worksheet 3 ln (k2/3.7 x 10-5)=1.359 e ln (k2/3.7 x 10-5)= e1.359 (k2/3.7 x 10-5)= 3.892 k2 =(3.892) (3.7 x 10-5) k2 = 1.44 x 10-4 s-1 5. If the activation energy of a reaction is 2.8x104 J/mol and has a rate constant of 0.143s-1 at 1400K, what is the rate constant of the reaction if the reaction occurs at a temperature of 1500K? ln(k2/k1)= Ea/R (1/T1-1/T2) ln(k2/0.143)= (2.8x104/8.314) (1/1400 -1/1500) ln(k2/0.143) = .1604 e ln(k2/0.143) = e.1604 (k2/0.143) = 1.174 k2 = .168 s-1 6. If the activation energy of a reaction is 3.45x103kJ/mol and has a rate constant of 0.567 s-1 at a temperature of 56 oC, what is the temperature, in Celsius, if the reaction has a rate constant of .896 s-1? For this problem, convert 3.45x103kJ/mol to J/mol, which would be 3450000 J/mol. ln(k2/k1)= Ea/R (1/T1-1/T2) ln(.567/.896) = (3450000/8.314) (1/T1 – 1/329) -.4576 = 414962.7 (1/T1 – 1/329) -1.1027 x10-6 = (1/T1 – 1/329) .00304 = 1/T1 1/.00304 = T1 329.12K =T1 329.12-273= 56.12 oC 7. If the slope of the following graph is -7.8x104, what is the activation energy of the following reaction? Worksheet 3 ln(k) CH 117 Fall 2015 1 2 3 1/T 4 5 For an ln(k) vs 1/T graph, look at the Arrhenius equation ln(k) = -Ea/RT + ln(A) this equation is very much like a linear y=mx +b where y= ln(k), m= (-Ea/R) x= (1/T), and b= ln(A). For this reason the slope of the graph is equal to –Ea/R. In order to find the activation energy of the reaction, use the following steps: Slope= -Ea/ R -7.8x104 = -Ea/ 8.314 9381.8 J/mol = Ea