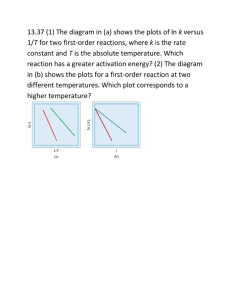
Rates, Temperature & Energy Diagrams Chemistry Problems
advertisement
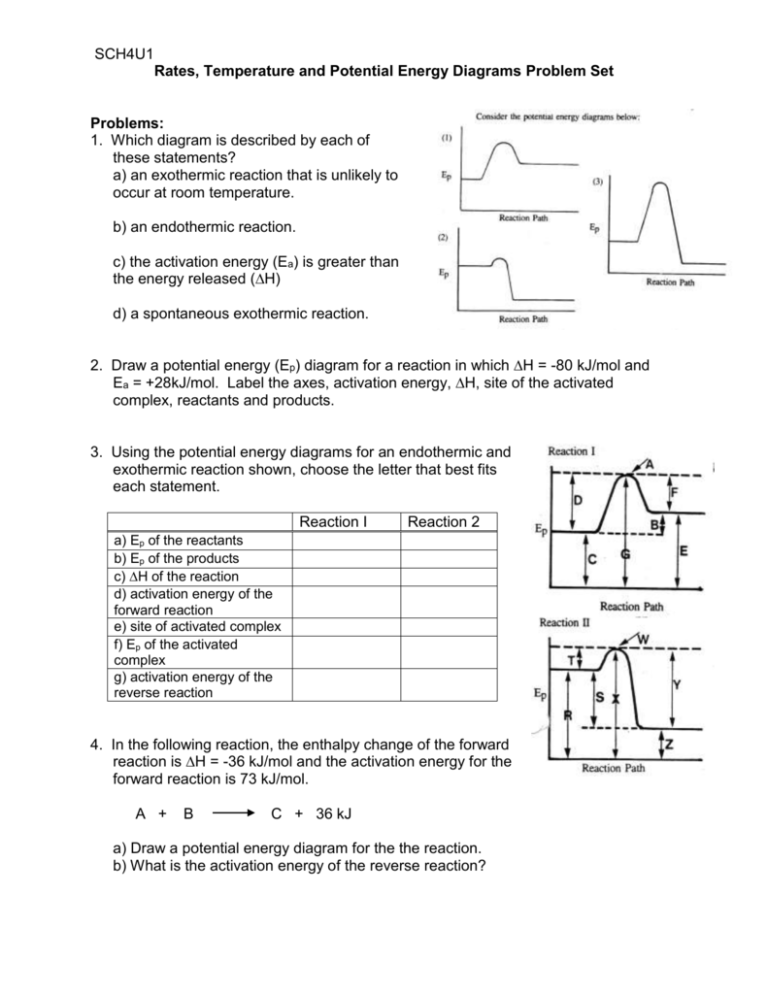
SCH4U1 Rates, Temperature and Potential Energy Diagrams Problem Set Problems: 1. Which diagram is described by each of these statements? a) an exothermic reaction that is unlikely to occur at room temperature. b) an endothermic reaction. c) the activation energy (Ea) is greater than the energy released (∆H) d) a spontaneous exothermic reaction. 2. Draw a potential energy (Ep) diagram for a reaction in which ∆H = -80 kJ/mol and Ea = +28kJ/mol. Label the axes, activation energy, ∆H, site of the activated complex, reactants and products. 3. Using the potential energy diagrams for an endothermic and exothermic reaction shown, choose the letter that best fits each statement. Reaction I Reaction 2 a) Ep of the reactants b) Ep of the products c) ∆H of the reaction d) activation energy of the forward reaction e) site of activated complex f) Ep of the activated complex g) activation energy of the reverse reaction 4. In the following reaction, the enthalpy change of the forward reaction is ∆H = -36 kJ/mol and the activation energy for the forward reaction is 73 kJ/mol. A + B C + 36 kJ a) Draw a potential energy diagram for the the reaction. b) What is the activation energy of the reverse reaction? 5. The activation energy of a forward and reverse reaction are as follows: i) C2H4 (g) + H2 (g) ii) C2H6 (g) C2H6 (g) C2H4 (g) + H2 (g) Ea = 180 kJ/mol Ea = 317 kJ/mol a) Draw a potential energy diagram for this reversible reaction. b) Calculate the enthalpy change (∆H) for each reaction. 6. Compare these reactions: i) C2H5Cl (l) C2H4 (g) + HCl (g) ii) C2H5Br (l) C2H4 (g) + HBr (g) Ea = 254 kJ/mol Ea = 219 kJ/mol Which of these two reactants would decompose more rapidly under the same reaction conditions and temperature? Explain your response. 7. A reaction consists of two steps as follows: Step 1: Step 2: A + B A + E C + E 2D Ea (kJ/mol) + 45 + 80 ∆H (kJ/mol) - 72 +28 a) Write the overall reaction equation. b) What is meant by the “rate-determining step”? c) Which of these steps is the rate-determining step? Why? d) What is the effect on the overall rate of increasing the concentration of A? What is the effect on the overall rate of increasing the concentration of B? Explain. 8. Examine the two Boltzmann distributions showing the distribution of kinetic energy possessed by the reactant molecules in two different reactions and the activation energy (Ea) for each reaction. a) Which reaction will be fastest at room temperature? Explain b) When the temperature eis increased to 60oC, what will happen to the rate of (i) Reaction 1 and (ii) Reaction 2? Why? c) In which case does the activation energy requirement change when the temperature is increased? 9. For the reaction: H2 + Cl2 2 HCl + 184 kJ the activation energy for the process is 156 kJ/mol. What is the activation energy for the decomposition of HCl to produce H2 and Cl2? 10. Using the concepts of kinetic energy and surface area, explain why sugar cubes can be used in hot coffee but granulated sugar is preferable for making iced tea. 11.Given the kinetic energy distribution curves and threshold energy (Ea) for reactions A and B: a) Which reaction will be faster at room temperature? b) Which reaction will show the greatest increase in the rate of reaction if the temperature is increased?