Answers - Chemistry Courses: About
advertisement
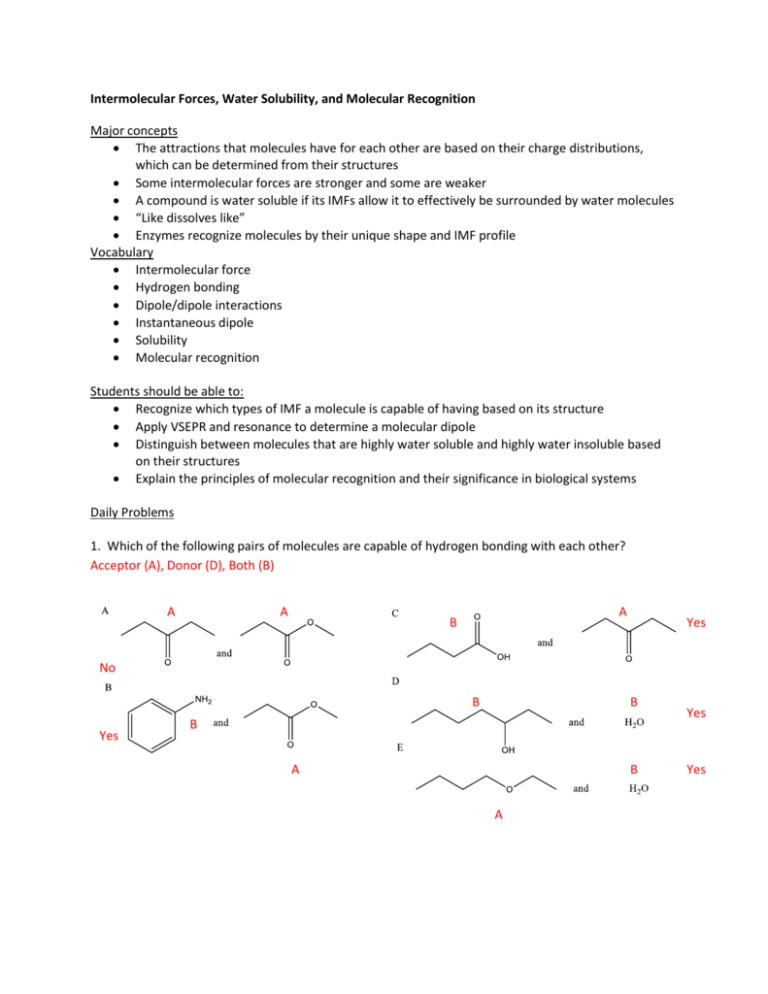
Intermolecular Forces, Water Solubility, and Molecular Recognition Major concepts The attractions that molecules have for each other are based on their charge distributions, which can be determined from their structures Some intermolecular forces are stronger and some are weaker A compound is water soluble if its IMFs allow it to effectively be surrounded by water molecules “Like dissolves like” Enzymes recognize molecules by their unique shape and IMF profile Vocabulary Intermolecular force Hydrogen bonding Dipole/dipole interactions Instantaneous dipole Solubility Molecular recognition Students should be able to: Recognize which types of IMF a molecule is capable of having based on its structure Apply VSEPR and resonance to determine a molecular dipole Distinguish between molecules that are highly water soluble and highly water insoluble based on their structures Explain the principles of molecular recognition and their significance in biological systems Daily Problems 1. Which of the following pairs of molecules are capable of hydrogen bonding with each other? Acceptor (A), Donor (D), Both (B) A A A B Yes No B Yes B B A B A Yes Yes 2. Which of these molecules has/have a molecular dipole moment? Draw an arrow to represent the dipole moment(s) or write “no molecular dipole.” no molecular dipole.. they cancel Overall molecular dipole: individual bond dipoles add up or cancel each other out in molecular dipole 3. Why is it not possible to rank Hydrogen bonding as always a stronger or weaker intermolecular force than van der Waals (induced dipole) interactions? Even though van der Waals forces are weak, they can add up. In this example, the many van der Waals forces between hydrocarbons are overall more powerful than one H-bond. 4. Why is it incorrect to say that “a water molecule has hydrogen bonding.” What is a better way to say what you mean? We can never talk about just one molecule with intermolecular forces. To have intermolecular forces, there must be multiple molecules to interact. Better to say that two water molecules are capable of Hbonding to each other. 5. Would you expect these molecules to be water soluble or water insoluble? no no “like dissolves like” water soluble: -ionic yes yes -highly polar -many H-bond acceptors and donors no Cumulative problems 6. You might expect this compound to have a dipole moment based on its Lewis Dot structure, but it doesn’t. Explain, using its “real” electron distribution. If we just consider this structure, the molecular dipole would be as shown. If we consider the resonance hybrid, the dipoles cancel out. 7. When we say that the nucleic acid bases guanine and cytosine “base pair”, we are saying that they recognize each other with complementary intermolecular forces, which hold them together, like a magnet. Below are their structures , aligned in the proper orientation to each other. Using your knowledge of resonance , redraw them in the more aromatic looking structures. Indicate partial charges on the base pairing atoms to show how they align so strongly. Notice how the acceptors are electron rich and the donors are electron poor. Also notice the perfect alignment to maximize closeness of all H-bonding partners. 8. Material Science: Teflon is the “non-stick” polymer that coats many frying pans. Most compounds are either more attracted to aqueous-like compound or hydrocarbon-like compounds. Teflon, on the other hand, does not stick to oily (hydrocarbon) substances, and water beads up on the surface because of its bad intermolecular interactions with Teflon. Explain why Teflon is both hydrophobic and lipophobic based on its structure: F F F F F F F F F F F F F F F F F F F F F F F F F F Hydrophobic: fluorine has lone pairs that hold closely to the nucleus as it is the most electronegative atom. This makes it a bad H-bond acceptor, so water does not form any H-bonds. Lipophobic: fluorine lone pairs are so stabilized to the nucleus that no instantaneous dipoles exist, leading to minimal van der Waals forces with non polar molecules such as oils. 9. Nutrition: Vitamins are labeled as “water-soluble” or “fat-soluble” based on their storage in the body. Water soluble vitamins are excreted quickly in the urine; fat-soluble vitamins are stored for longer periods in body-fat reserves and therefore do not need to be consumed daily. Based on structure alone, it is possible to determine fat vs. water solubility. Which of the following molecules is/are most likely to be excreted quickly? HO OH O H OH vitamin A N Vitamin B6 O OH HO NH2 O excreted quickly= water soluble N HO Vitamin B3 OH Vitamin C O Vitamin K R O 10. Enzymes: Enzymes are proteins, which are large molecules made up of many amino acids. Amino acids contain a variety of functional groups. Enzymes bind to smaller molecules through the intermolecular forces we have been discussing. The way that enzymes “recognize” which small molecules to bind to is through having complementary IMFs in their active sites. In the figure below--taken from Proceedings of the National Academy of Science, 97,2011-2016 (Feb 2000) by Wolfenden and coworkers--the small molecule is in red and the groups from the enzyme are in black. The interactions between the enzyme and molecule are given by colored dashed lines, and the interactions between the protein and other parts of the protein are given in black dashed lines. Describe the types of IMFs observed. How does this set of IMFs make this enzyme very selective for binding this small molecule rather than any other? * * * / * * / * * * Overall, the molecule fits perfectly in the pocket (active site) so that is excludes water and maximizes IMF. * * = H-bonds / = ionic interaction Extension problem 11. Water can hydrogen bond to acetic acid (vinegar) by placing its most negative area (lone pair) next to the most positive area (hydrogen atom) of acetic acid. This forms a fairly strong interaction, which is close to becoming a bond. What if the interaction became a full bond, and the existing H-O bond broke, as illustrated by arrows? What would the products be? δ- δ+