Intermolecular forces - Keith Grammar School
advertisement

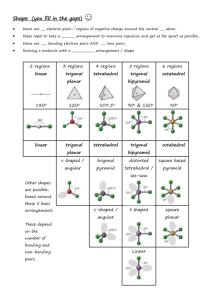
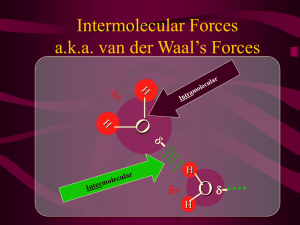
Higher Chemistry Unit 1(d) Intermolecular forces of attraction After today’s lesson you should be able to: Explain the difference between intramolecular and intermolecular forces of attraction. Explain what is meant by a permanent dipole, temporary dipole and induced dipole. Give examples of where these dipoles are found. Explain where hydrogen bonding occurs. State the relative strengths of each intermolecular force. What are intermolecular forces? Intermolecular forces are the weak forces of attraction that occur between covalent molecules and the monoatomic gases when thy are in their liquid and solid states. They influence the physical properties of molecules e.g. melting and boiling points, solubility in water and other solvents, viscosity (the flow rate) and the density. There are three types of intermolecular attractions: 1. Temporary dipole – induced dipole (Van der Waal attractions) 2. Permanent dipole – permanent dipole 3. Hydrogen bonds Temporary dipoles Occur in all non- metal atoms and molecules. Occur due to constantly moving electrons around the nucleus; this results in the electrons being unevenly distributed. Occasionally there are more electrons on one side of the atom or molecule than the other. no dipole temporary dipole This results in the atom or molecule having a slightly positive side and a slightly negative side. Only occur for a split second. Induced dipoles Occur when a permanent or temporary dipole induce (force) the electrons in another unpolarised atom or molecule to move to one side resulting in a dipole. Electrons can be attracted or repelled within the atom being induced. Permanent dipoles A polar covalent bond is a permanent dipole because one atom has a permanent δ- charge whereas the other a permanent δ+ charge. e.g. HCl, H2O, NH3. polar covalent bond OR permanent dipole Van der Waal’s attractions Weakest intermolecular force Occur between ALL non-metal atoms and molecules. Strongest for large atoms and large molecules because both have lots of electrons which can form temporary dipoles. Permanent dipole – permanent dipole Additional to Van der Waals’ forces Stronger than Van der Waals’ forces Occur between polar molecules. The slightly positive charge of one molecule is attracted to the slightly negative charge of another and vice versa. permanent dipole permanent dipole permanent dipole to permanent dipole attraction Hydrogen bonding Occurs between molecules which have a hydrogen atom directly bonded to a nitrogen, oxygen or fluorine atom. Additional to Van der Waals’ forces and permanent dipole-permanent dipole interactions Strongest intermolecular force Exercise What type of dipole would be present in a molecule of each of the following? Hydrogen fluoride Iodine Methane Carbon dioxide What type of intermolecular force(s) would occur between molecules of the following? Hydrogen fluoride Chlorine Poly(ethene) Water Molecules containing polar covalent bonds and symmetry If a molecule contains polar covalent bonds (permanent dipoles) and is symmetrical the charges are cancelled out and the overall molecule is non-polar. The intermolecular forces will not be permanent dipole-permanent dipole attractions but Van der Waals’ forces. e.g. If a molecule containing polar bonds is non-symmetrical then the charges do not cancel out and the overall molecule is polar. The intermolecular forces will be Van der Waals’ forces and permanent dipolepermanent dipole attractions.