Types on intermolecular forces.
advertisement
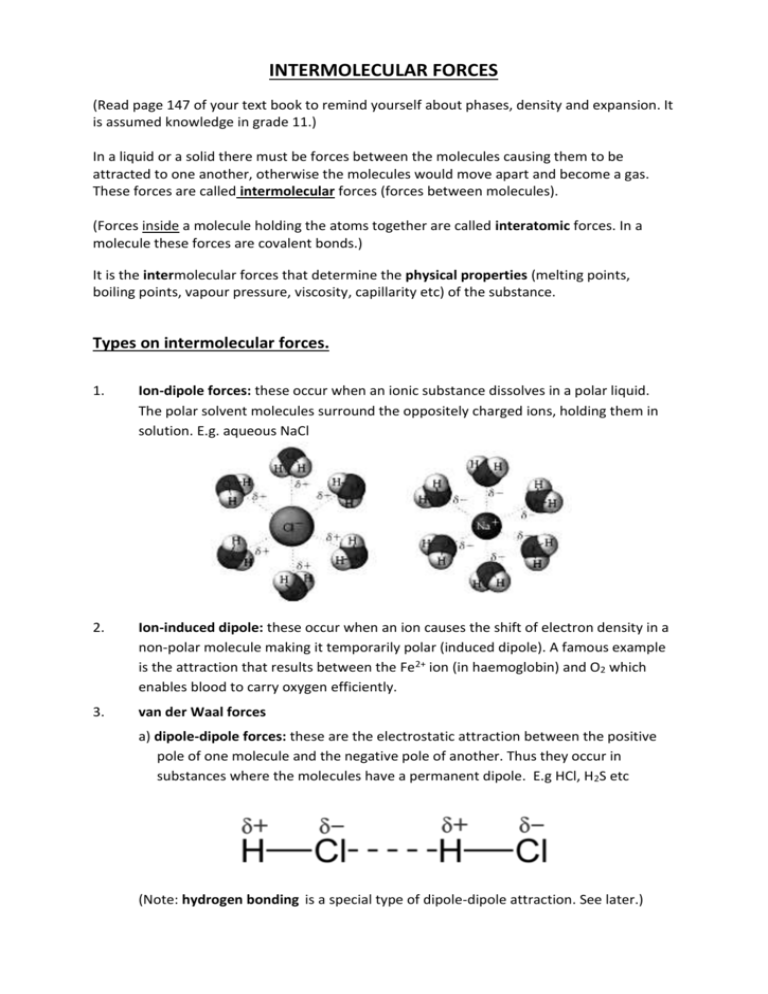
INTERMOLECULAR FORCES (Read page 147 of your text book to remind yourself about phases, density and expansion. It is assumed knowledge in grade 11.) In a liquid or a solid there must be forces between the molecules causing them to be attracted to one another, otherwise the molecules would move apart and become a gas. These forces are called intermolecular forces (forces between molecules). (Forces inside a molecule holding the atoms together are called interatomic forces. In a molecule these forces are covalent bonds.) It is the intermolecular forces that determine the physical properties (melting points, boiling points, vapour pressure, viscosity, capillarity etc) of the substance. Types on intermolecular forces. 1. Ion-dipole forces: these occur when an ionic substance dissolves in a polar liquid. The polar solvent molecules surround the oppositely charged ions, holding them in solution. E.g. aqueous NaCl 2. Ion-induced dipole: these occur when an ion causes the shift of electron density in a non-polar molecule making it temporarily polar (induced dipole). A famous example is the attraction that results between the Fe2+ ion (in haemoglobin) and O2 which enables blood to carry oxygen efficiently. 3. van der Waal forces a) dipole-dipole forces: these are the electrostatic attraction between the positive pole of one molecule and the negative pole of another. Thus they occur in substances where the molecules have a permanent dipole. E.g HCl, H2S etc (Note: hydrogen bonding is a special type of dipole-dipole attraction. See later.) b) dipole-induced dipole forces: these occur when a polar substance is mixed with a non-polar substance. The polar molecule induces a polarity in the non-polar molecule which then results in an electrostatic attraction between the two molecules. e.g HCl mixed with Cl2 c) induced dipole-induced dipole forces (London Forces): these occur between nonpolar molecules. Electrons are mobile in the orbitals of a molecule so that at any given moment it is possible that there is an uneven distribution of electrons in the molecule giving it a temporary dipole. This momentary dipole can induce a temporary dipole in a neighbouring molecule resulting in a temporary weak attraction known as a London Force. London forces are greater the greater the mass of the molecule. compound CH4 C2H6 C3H8 C6H14 C23H48 Relative molecular mass 16 30 44 86 323 Boiling point oC -162 -89 -42 68 380 Note: other factors, including the shapes of molecules, also affect the physical properties if a compound. d) hydrogen bonding is a special type of dipole-dipole attraction and are only found when: i. hydrogen is covalently bonded to small and highly electronegative nitrogen, oxygen or fluorine. ii. there is at least one lone pair on the O, N or F. e.g. HF, H2O, NH3, (plus all alcohols and carboxylic acids) The electronegative (F, O or N) atom largely the hydrogen of its electron which results in a highly positive hydrogen. This positive hydrogen buries itself in the lone pair of a neighbouring molecule forming a short, directional and strong intermolecular bond. Hydrogen bonds result in higher than expected intermolecular forces. e.g. Boiling points of hydrogen halides: Looking at the trend below you would expect the b.p. of HF to be about -100oC but instead it is +19oC. This is as a result of a strong Hydrogen Bond. Boiling point oC HF 19 HCl -85 HBr -67 HI -35 It is the strong hydrogen bonding which makes water (a tiny molecule) a liquid at room temperature. (The fact that ice floats on water is a result of hydrogen bonding being directional. As ice freezes it forms a more open structure.) water ice Some terms you need to know...................... Viscosity: the resistance of a liquid to flow. The viscosity is dependent upon the temperature but also upon the strength of the intermolecular bonds in the liquid. THE STRONGER THE INTERMOLECULAR BONDS THE MORE VISCOUS THE LIQUID. Compound Viscosity (mPa.s) water 0,89 methanol 0,55 glycerol 1200 corn syrup 1380 mercury 1,53 Evaporation: the change of a liquid into a vapour below its boiling point. THE STRONGER THE INTERMOLECULAR BONDS THE LOWER THE RATE OF EVAPORATION. Compound water Relative evaporation 1 ethanol 1,6 acetone 11 Ethyl ether 13 A liquid which evaporates readily is called a volatile liquid. Melting and boiling points: In order for a substance to melt intermolecular bonds must be weakened sufficiently for a liquid to form. In order for a liquid to boil intermolecular bonds must be weakened sufficiently for the molecules to completely separate from one another. THE STRONGER THE INTERMOLECULAR BONDS THE HIGHER THE MELTING AND BOILING POINTS. Surface tension: a property of liquids caused by intermolecular forces near the surface leading to the apparent presence of a surface film. THE STRONGER THE INTERMOLECULAR BONDS THE HIGHER THE SURFACE TENSION. Compound Surface tension mN.m-1 at 20 oC water 74 ethanol 22 Acetone 24 glycerol 63 Sunflower oil 31 Capillarity: The tendency of a liquid in a capillary tube or absorbent material to rise or fall as a result of surface tension. The greater the surface tension the greater the rise or fall of the liquid. Note the shape of each meniscus. water mercury











