HW 1-C1&2-Sp13 - Louisiana Tech University
advertisement

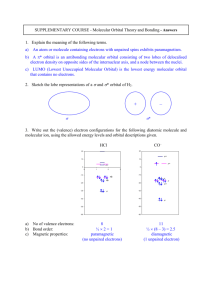
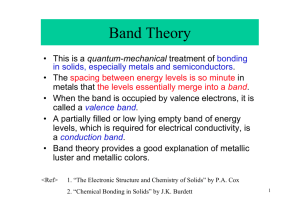
HW 1. CHEM 481 Chapters 1 Chapter 1. Name:________________________ 1. What are the two basic types of nuclear reactions? Give examples of each that occur during the formation of the Universe 2. Complete the following Nuclear reactions: a) Uranium – 238 decays by alpha radiation to produce what other element? b) Thorium – 234 decays by beta radiation to what other element? c) What element did we start out with if the result of beta decay is bismuth– 214? d) What element is produced when mercury – 201 captures an inner shell electron with the production of a gamma ray to release excess energy? 3. Predict the most likely modes of decay and the products of decay of the following nuclides: (a) 17F: (b) 105Ag: (c) 185Ta: 4. Using the binding energy calculator, calculate the binding energy 235U if the mass of the this nuclide (isotope) is 235.0349 amu. ( P= 1.007277 amu, N= 1.008665 amu) 5. What are theories that have been used to describe the nuclear stability? 6. How long would it take for a sample of 222Rn that weighs 0.750 g to decay to 0.100 g? Assume a half-life for 222Rn of 3.823 days? 7. The skin, bones and clothing of an adult female mummy discovered in Chimney Cave, Lake Winnemucca, Nevada, were dated by radiocarbon analysis. How old is this mummy if the sample retains 73.9% of the activity of living tissue? 8. Describe the Schrödinger equation and the breaking up of wave function, into radial and angular component of a wave function and explain the general rule used to find the number of radial and angular nodes of a wave function. 9. Using Bohr energy calculator, calculate the wavelength of light that can excite the electron in a ground state hydrogen atom from n = 5 to n = 3 energy level. 10. Consider the following radial probability density-distribution plot and respond to the associated questions. a) How many radial nodes are there? b) If the total number of nodes is 3, what type of orbital is involved? c) Which orbital would it be if there were one more node? 11. Cu: (1s2)(2s2, 2p6) (3s2,3p6) (3d10) (4s1) : there are two possible scenarios for forming Cu+ ion- ionizing 3d10 electron or 4s1. Using Slater’s Rules show which one of the electrons would come out easily. 12. Give the ground state electronic configurations of following in core format. a) Mo b) Ag c) V3+ d) Mn2+ e) Cr2+ f) Co3+ g) Cr6+ h) Gd3+ 13. Give the ground state electronic configurations of the following in valence orbital box format and give the number of unpaired electrons. a) Mn: b) Co: c) Fe2+: d) Nd3+: 14. Why is the ionization energy of P (11.00 eV) greater than S (10.36 eV)? 15. How do you measure atomic (ionic) radii (size)? 16. Why the atomic radius of Zr (1.64) which is in 5th period is almost similar to a element, Hf (1.65) in 6th period. 17. How you define electronegativity? 18. Calculate the electronegativity (X) (Xm, Xar) for Cl. [ Xm= 1/2(I+Ae); Xar= 0.744+ 0.359 Zeff/r2 ] HW 1. CHEM 481 Chapters 2 Chapter 2. Name:________________________ 1. Draw Lewis structure for SbF5, ClF3, and IF6+: 2. Predict geometry of central atom using VSEPR and the hybridization in problem 1. 3. Why hypervalent compounds are formed by elements such as Si, P and S, but not by C,N and O? 4. Using valence-bond (VB) theory to explain the bonding in the coordination complex ion, Co(NH3)63+. 5. What is the oxidation state of metal in (a) Co(NH3)63+ ion (b) PtCl42- ion. 6. Draw a diagram to illustrate each described overlap: a) bonding overlap of two p orbitals b) bonding overlap of two d orbitals c) bonding overlap of a p orbital and a d orbital d) antibonding overlap of a p and a d orbital e) antibonding overlap of two d orbitals. 7. Using molecular orbital theory and diagrams, explain why, O2 is a paramagnetic whereas N2 is diamagnetic. 8. Draw molecular orbital diagrams for HF, CO, NO, NO+. Calculate their bond order and predict magnetic properties. 9. Draw a molecular orbital diagram for triangular H3+ and describe the bonding. 10. Draw a Walsh diagram (orbital correlation diagram) and show that triangular H 3+ is more stable than linear H3+. 11. Using molecular orbital diagrams for pi (p) orbitals explain the relative stabilities of the following: (a) C3H3 and C3H3+ (b) C4H4 and C4H4+ (c) C5H5 and C5H5(d) C6H6 and C6H6+ (e) C7H7 and C7H7+ 12. Pick the isolobal fragments among the following: a) Co3(CO)9Co(CO) 3, Co3(CO)9PR, Co3(CO)9CH b) H3CCl, Mn(CO)5H, Re(CO) 5Cl c) R2SiH2, Fe(CO)4H2, H2CH2 13. Describe metallic bonding and properties in terms of: a) Electron-sea model of bonding: b) Band Theory: 14. Draw the s band (molecular orbitals) for ten Na on a line (one dimensional) and show bonding and anti-bonding molecular orbitals and fill electrons. 15. Describe the metallic properties of sodium in terms of band theory. 16. Using a band diagram, explain how magnesium can exhibit metallic behavior even though its 3s band is completely full. 17. Draw a Band diagram for carbon/silicon/germanium/tin, and label valence band, conduction band and band gap? 19. Draw a band diagrams to show the difference between(Band gaps: C = 5.47, Si = 1.12, Ge = 0.66, Sn = 0) a) Conductor (Sn): b) Insulator (C): c) Semiconductor (Ge): 20. Draw a band diagram for thermal/photo (Intrinsic) and doped (Extrinsic) semiconductors and explain the origin of semicondictivity? d) Thermal/photo (Intrinsic) (Ge): e) Doped (Extrinsic) (Si/As): 21. Draw a band diagram for a p-type (Si/Ga) and n-type (Si/As) semiconductors and show holes and electrons that is responsible for semiconductivity. f) p-type(Si/Ga): g) n-type(Si/As): 22. What is a transistor with emitter (E), collector(C) and base (B), and how it works? 23. What the difference between a transistor (semiconductor device) and vacuum tube? 24. Using the diagram explain how a diode work.