lab1 - blahblehblah
advertisement

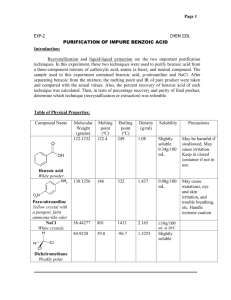
David Goldfarb Wednesday, February 03, 2010 CHEM-2290 Experimental Chemistry II Lab 1: Crystallization in Single and Mixed Solvent with and without Activated Charcoal Introduction: Solubility is based on the ability of one substance to dissolve another. The rule ‘like dissolves like’ is applied to solubility, polar solvents will easily dissolve polar solutes and likewise for non-polar substances. The method of crystallization and recrystallization is a way to purify substances. As a solution is heated, more solute will go into solution as the solution becomes saturated. On cooling, the compound’s solubility decreases leading to it crystallize (forming a mass of solid). These crystals are purer than the original substance because from one seed crystal of pure substance, only pure crystal will form. The impurities will remain in solution longer, or would have been removed prior to crystallization (they would be less soluble). There are many methods of crystallization, different solvents and solvent systems play a large role in the crystallization process. In this lab the solubility of benzoic acid was analyzed in various solvents, and then several crystallizations were carried out: impure benzoic acid in water, pure benzoic acid in toluene-hexane, naphthalene in methanol-water. A decolorizing test was also performed. Data and Results: Solubility of Benzoic Acid in Various Solvents At Room Temperature In Hot Solution Crystallization on Cooling Water Insoluble Slightly Soluble Yes Toluene Slightly Soluble Soluble Somewhat Hexane Insoluble Soluble Yes Mass of Impure Benzoic Acid used: 0.944g Mass of Pure Benzoic Acid used: 0.984g Mass of Naphthalene used: 2.058g Mass of Benzoic Acid from crystallization (in water): 0.379g (40.15% recovery [.379/.944 *100%]) Mass of Benzoic Acid from crystallization (in toluene-hexane): 0.271g (27.54% recovery [.279/.984*100%]) Mass of Naphthalene from crystallization: 0.3829g (18.61% recovery [.3829/2.058 *100%]) Discussion and Conclusion: It was obvious that benzoic acid was more soluble in some solvents than in others, but through the crystallizing procedure it was dissolved and purified. Though not a large amount of the product was recovered, it was quite pure. The samples dissolved and crystallized well during the procedure, there was some that did not but it was probably mostly impurities. This was removed by gravity filtration, so it makes up for some of the lost product. Based on the melting points of the final products, the crystals were significantly more pure and well-formed. The main mistake I made was just general rushing, due to lack of preplanning and inexperience, I wasn’t able to allow the reaction as much time as it needed. I also didn’t pay as much attention to recording data and observations as I should have; this led to some unclear ideas as to how well the experiment went. These are both problems that can be improved upon later, and do not reflect on this lab specifically. Despite these mistakes, the concepts addressed in the lab remained. The procedure of recrystallization for purification was clearly demonstrated. The Benzoic Acid and Naphthalene were successfully collected, and in appreciable amounts. Experiment 1 Questions: Melting Point of Purified Benzoic Acid (from water): 118°C. This is reasonably close to the established melting point of Benzoic Acid which is 122°C. This would indicate that the sample was reasonably pure. It was very close to the measured melting point of the crystalline product of the pure Benzoic Acid, this was 119°C. The Percent Recovery of the Pure Benzoic Acid was only 27.54%. This is significantly lower than the percent recovery of the impure Benzoic Acid. This could be because of the different solvent system used for this recrystallization. Benzoic Acid was more soluble in these solvents at room temperature. Therefore, as it cooled it did become less soluble, but not as insoluble as it did in water. This probably hindered crystallization to an extent. It’s also possible that some of the pure Benzoic acid stayed in solution during the filtration process, and did not precipitate or crystallize.