How Chemical Reactions Happen (INFORMATION PAGE)

How Chemical Reactions Happen
Chemical reactions happen when atoms give electrons to another atom, take
electrons from another atom, or share electrons.
Electrons orbit the nucleus of an atom in energy shells. For the most stable atom, the outer shell needs to be full of electrons. To get a full shell, atoms will give, take or share electrons with other atoms. This causes a chemical reaction and forms a chemical bond between the combined atoms. The new substance(s) formed will have different properties.
Here are some examples:
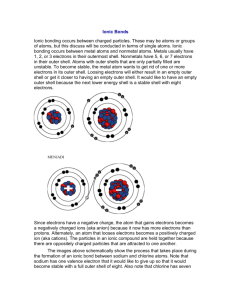
Sodium (Na) has 11 electrons:
2 filling its first shell, 8 more filling its second shell, and one all alone in its’ 3 rd shell.
To become more stable, sodium will easily give up that single electron so it will have a full shell. The extra electron will go to another atom that needs an electron to have a full shell.
Chlorine has 17 electrons, and needs another electron to complete its 3 rd energy shell to become stable. If it takes one electron from sodium it forms a bond between the atoms of sodium and chlorine, and creates a new compound; sodium chloride, or table salt.
If lithium gives one valence electron to florine, they stabilize one another and form lithium floride.
If one
calcium atom
gives an electron to each of
2 chlorine atoms, it
stabilizes all,
and creates
calcium
chloride
Chemical bonds formed when electrons are transferred from one atom to another are
ionic bonds
.
Oxygen is missing 2 electrons in its outer shell. By sharing electrons with another oxygen atom, it creates
O
2
and becomes stable.
Hydrogen atoms
lack one valence
electron. Oxygen
lacks 2. If one
oxygen atom combines with 2 hydrogen atoms, they stabilize one another, and form a water molecule.
Chemical bonds formed when electrons are shared between atoms are
covalent bonds
.