How Chemical Reactions Happen (INFORMATION)
advertisement

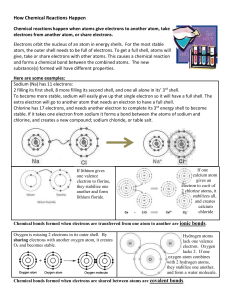
Chemical Reactions Chemical reactions happen when atoms give electrons to another atom, take electrons from another atom, or share electrons. Electrons orbit the nucleus of an atom in energy shells. For the most stable atom, the outer shell needs to be full of electrons. To get a full shell, atoms will give, take or share electrons with other atoms. This causes a chemical reaction and forms a chemical bond between the combined atoms. The new substance(s) formed will have different properties. Here are some examples: Sodium (Na) has 11 electrons: 2 filling its first shell, 8 more filling its second shell, and one all alone in its’ 3rd shell. To become more stable, sodium will easily give up that single electron so it will have a full shell. The extra electron will go to another atom that needs an electron to have a full shell. Chlorine has 17 electrons, and needs another electron to complete its 3rd energy shell to become stable. If it takes one electron from sodium it forms a bond between the atoms of sodium and chlorine, and creates a new compound; sodium chloride, or table salt. If lithium gives one valence electron to florine, they stabilize one another and form lithium floride. If one calcium atom gives an electron to each of 2 chlorine atoms, it stabilizes all, 3 and creates calcium chloride Chemical bonds formed when electrons are transferred from one atom to another are called ionic bonds. When an atom donates an electron, it becomes positively charged, because it now has more positively charged protons than negatively charged electrons. The atom that accepts the electron becomes negatively charged. These charged particles are called ions. Since opposite electrical charges attract, the ions bond to each other in a bond called an ionic bond. Ionic bonding generally takes place between a metal and a nonmetal. Sometimes, rather than exchange electrons, atoms simply share electrons. Chemical bonds formed when electrons are shared between atoms are covalent bonds. The prefix co- means together/jointly (as in cooperate ,copilot,costar) , so co-valent bonds are bonds from joined/shared valences.) Oxygen is missing 2 electrons in its outer shell. By sharing electrons with another oxygen atom, it creates O2 and becomes stable. Hydrogen atoms lack one valence electron. Oxygen lacks 2. If one oxygen atom combines with 2 hydrogen atoms, they stabilize one another, and form a water molecule. When atoms change their electrons, it causes chemical reactions which create new substances with different properties. They are not just a physical change. Physical Change Molecules stay the same. The size, shape or phase of a substance may change, but a new chemical is not formed. Ex: * water is still H2O if you freeze it. * paper is still paper if you crunch it up, or cut it into tiny pieces. Here are some changes that are physical: *Changes in size or shape *Changes in phase *Changes in density (for example, food coloring dropped in water becomes less dense, but the molecules of the food coloring and watert stay the same) Can be caused by cutting, grinding, bending, freezing, melting, mixing (if mixture parts of the mixture stay molecularly separate) Chemical Reaction Molecules change and a new substance with different properties is formed—The substance(s) present at the beginning of the reaction are no longer there at the end. Ex. *water that reacts with carbon dioxide becomes carbonic acid, and is not water or carbon dioxide any more. * paper isn’t paper if it has burned to ash Here are some signs that a chemical reaction may be happening: *Temperature changes from the reaction *Sound, light or odor is produced *Gas is produced (fizzes or bubbles) *Precipitate forms (solid formed from 2 liquids) *Color change (this may also happen in some physical changes) Here is a memory tool that may help you” Chemical reactions may leave you AGAPE. __Altered molecules __Gas formed __Aroma, color changes __Precipitate formed __Energy released/absorbed Can be caused by burning, rusting, cooking, fermentation,