Week 4 science homework Role of electrons
advertisement

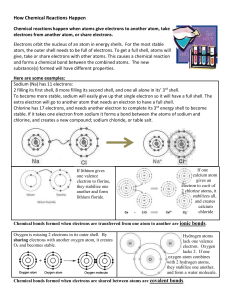
Week 4 science homework Role of electrons 1.Define the term ‘energy levels’. Electrons orbit within their designated shell or energy level. 2.Identify the number of electrons each shell normally holds. First shell hold 2, second, third and fourth holds normally 8 electrons. 3. Clarify what the electronic configuration of an atom shows. Electronic configuration is how the electrons fit into the shells. For example Calcium has 20 electrons, in the first shell there would be 2 electrons in the next two shells there would be 8 electrons. 4.Describe the electronic configuration of magnesium. Magnesium has 12 electrons so the first two would go into the first shell, another eight would fill the second and the left over two would go in the third shell 5. Describe what the following have in common: a. atoms in the same group. The group number represents the number of electrons in the outer shell. b. atoms in the same period they have the same number of electrons in the outermost shell. 6. Identify which group contains elements that rarely react. Group 8 7. Distinguish between atoms that react and atoms that don’t react. Atoms that react don’t have their outside shell filled where as the noble gases do and are stable. 8. Compare a chlorine atom with a chloride ion. Chlorine is a naturally occurring element with a symbol Cl and atomic number 17. Under standard conditions. Chlorine atoms gain one electron to become a chloride ion (Cl−) 9. Describe what happens when a sodium ion forms. Sodium combines with chlorine and loses its outside shell and becomes positively charged. Chlorine takes one from sodium to form and becomes negative when the combine they form sodium chloride. 10. Explain the difference between the formation of a positive ion and a negative ion. The formation of a positive electron is when an atom loses an electron or outside shell to another type of atom. Negative is when they take an electron from another atom. 11. Identify three positive and three negative ions by name and symbol. 12. Explain why noble gases do not form ions. They don’t form ions because they are a complete atom with full electron configuration and shells. They don’t need to take any and because they are complete they can’t give any. 13 Sodium chloride has charges but no overall charge. Explain.