5.111 Principles of Chemical Science MIT OpenCourseWare Fall 2008 rms of Use, visit:
advertisement

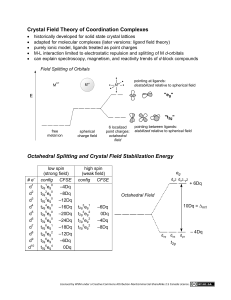
MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 29.1 5.111 Lecture Summary #29 Transition Metals Topic: Crystal Field Theory Continued, and Magnetism Chapter 16 p 631-637 Magnetism Compounds possessing unpaired electrons are paramagnetic (attracted by magnetic field); those in which the electrons are paired are diamagnetic (repelled by magnetic field). Crystal Field Theory: Octahedral Case (See Lecture #28) Crystal Field Theory: Tetrahedral Case The tetrahedral crystal field splitting energy (∆T) is smaller than for octahedral complexes because the point charges are not directed at any orbital set. z axis L Z Z L Y y axis M X X L L- Y x-axis out of the page dz2 dx2-y2 tetrahedral z axis L Z L M Z Z y axis L Lx-axis out of the page Y Y X X dyz Y X dxz dxy tetrahedral Geometry of tetrahedral complexes result in greater orbital destabilization for dyz, dxz, dxy than for dx2-y2 and dz2 (opposite of octahedral). There is more repulsion between the ligand negative point charges and the d-orbitals that are 45° off axis (dyz, dxz, dxy) than there is between the ligand negative point charges and the d-orbitals that are on axis (dz2 and dx2-y2). dyz, dxz, dxy have the same energy with respect to each other (degenerate). dz2 and dx2-y2 have the same energy with respect to each other (degenerate). 29.2 E (eg) dz2 dx2-y2 O +3 5 O dxy (Spherical crystal field) O 5 dxy dxz dyz dyz + 2 5 T -2 average energy of d orbitals with ligands dxz (t2) dx2-y2 dz2 (e) - 3 5 (t2g) (Tetrahedral crystal field) (Octahedral crystal field) ∆T is the tetrahedral crystal field splitting energy Again, ∆T is smaller than ∆o because the point charges are not directed at any orbital set in a tetrahedral crystal field. Because ∆T is small, many tetrahedral complexes are high spin. You can assume that they are all high spin. Because the overall energy in the tetrahedral crystal field is maintained, t2 orbitals (dxy, dxz, and dyz) go up in energy by 2/5, and the e orbitals (dx2-y2 and dz2) go down in energy by 3/5. Tetrahedral Example for Cr3+ (a) figure out d electron count (b) draw tetrahedral crystal field splitting diagram, label orbitals, and fill in electrons E (t2) T (e) average energy of d orbitals with ligands (Spherical crystal field) (Tetrahedral crystal field) (c) Write dn electron configuration: (d) How many unpaired electrons? T T 29.3 Crystal Field Theory: Square Planar Case Z-axis Z Z L L- Mn+ L- Y-axis Y X X L Y dz2 dx2-y2 X-axis Square planar lots of repulsion ligand point charges directed at orbitals Destablized compared to all other d-orbitals Z Z-axis much less repulsion than in octahedral crystal field. Less repulsion than for dx2-y2 and for dxy Z Z L L- Mn+ L- LX-axis Square planar Y-axis X X dyz Y Y Y X dxz dxy stabilized compared stabilized compared more repulsion than for to dxy and dx2-y2 to dxy and dx2-y2 dyz, dxz, and dz2. Less repulsion than for dx2-y2 since orbitals are 45° off axis in dxy. 29.4 dx2-y2 dx2-y2 E (eg) dz2 +3/5 O dxy O average energy of d-orbitals with ligands (Spherical crystal field) dxy dxz -2/5 (t2g) dyz O dz2 (Octahedral crystal field) dyz dxz (Square planar crystal field) The overall energy of the square planar crystal field is maintained as well, but the relative energies of each of the d-orbitals are more complicated and you are not expected to know them. Putting it all together: If a Ni2+ (d8) center in an enzyme is found to be diamagnetic, does it have square planar, tetrahedral, or octahedral geometry? dx2-y2 E dx2-y2 dz2 dxy dxy dxz dyz (Octahedral crystal field) dz 2 dxz dyz dxy dx2-y2 dxz dyz dz2 (Tetrahedral crystal field) (Square planar crystal field) Answer: An example of a square planar Ni site in Nature is found in enzyme called acetyl-CoA synthase.