CE revision outline
advertisement

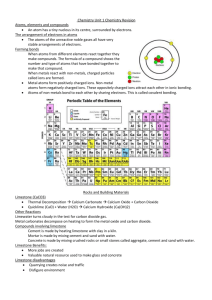
Common Entrance Revision Guide Main topic summary (Words in bold type indicate Level 2 only) Chemistry 1 (Materials and their properties) 2 The variety of materials The Bunsen Burner Know the main properties of and a use for a variety of everyday substances. How to light it, types of flame, structure, safety requirements 3 Making a solution Know that when a soluble solid is added to a suitable solvent then a solution is formed Terms solvent, solute, solution, soluble, insoluble and saturated solutions. Knowhow to show the effect of different temperatures and different solutes on the rate of dissolving. 4 5 Separating mixtures Acids and alkalis Know that Ethanol and propanone are alternative solvents to water Knowledge of filtration to remove insoluble solids from a suspension Simple distillation to obtain a solvent from a solution eg fresh water from blue ink or seawater. (how to prevent suck-back when distilling) Fractional distillation to recover ethanol from wine or beer Evaporation to obtain a solute from a solution Paper chromatography to separate coloured dyes. Know how to interpret a chromatogram Be able to draw a diagram of the apparatus used Know how to separate two solids eg chalk from salt Purifying rock salt Know the terms filtrate and residue Know how to use a Liebig condenser, filter funnel and mortar and pestle the differences between sea, tap and distilled water, demonstrated by evaporation; Knowledge of universal indicator, pH numbers and some common acids and alkalis. Applications of neutralisation in and medicine and agriculture Neutralization alt formation by the reaction between sodium hydroxide and hydrochloric acid 6 The air That air is a mixture of gases. the approximate percentages of nitrogen and other gases in the air; Be able to show that oxygen is 20% of the air (by rusting experiment or heating air with copper or another metal) That carbon dioxide is a product of respiration and a raw material for photosynthesis Be able to show that air contains water vapour using anhydrous cobalt chloride or anhydrous copper sulphate the uses of oxygen; When things burn in air they react with oxygen. Glowing splint test for oxygen. Limewater test for carbon dioxide The effect of burning fossil fuels (production of acid rain, carbon dioxide and solid particles) that air is often polluted by sulphur dioxide and carbon monoxide and the sources of these pollutants Show that water and oxygen is needed for rusting to take place. Carbon dioxide in the air dissolves in rain water to form acid rain 7 Physical change Evaporation, sublimation, condensation, melting and boiling points. Be able to make predictions about amount of water lost during evaporation The applications of evaporation and condensation in the water cycle 8 Chemical change Terms Burning, oxidation, Reduction, thermal Decomposition, Neutralization. The use of carbon to reduce various oxides Thermal decomposition of copper carbonate and potassium permanganate. When copper or magnesium is heated it oxidises and gains in mass How to recognise that a chemical change has taken place by a change in colour or change in temperature 1 Chemical change (Continued) Change in mass when chemicals are heated (eg heating magnesium or copper in air) Conservation of mass; Mass of reactants = mass of products (eg mix lead nitrate and sodium iodide to produce insoluble lead iodide) The combustion of methane and other fuels and that they produce carbon dioxide and water as products of combustion How to identify the products of combustion when a candle is burnt (Water using anhydrous cobalt chloride, carbon dioxide using limewater) 9 Elements and compounds Elements cannot be decomposed. Elements can combine together. Each element is made of a single kind of atom which is represented by a symbol Elements are organised into the periodic table What happens when elements react with oxygen (Carbon, copper, iron, magnesium, sulphur and zinc are examples for experiments on burning the elements in air and testing the oxides. Know that the properties of the compounds formed when elements combine are different to those of the constituent elements Meaning of words atom and molecule Know these symbols: H, C, O, N, S, Mg, Na, Cl, Ca, Cu, Fe and He; Two elements can combine to form a compound. (eg Iron and sulphur, copper and oxygen) Differences between a metal and non-metals using various properties: (Electrical conductivity, shininess, malleability and whether they give acidic or basic oxides) know how to write word equations know some simple formulae H2O, CO2, O2, CH4, NaCl, HCl, NaOH, CaCO3 10 Activity series Reduction and oxidation. Displacement of one element by another using reactions between metals and solutions of the sulphates of other metals. Extraction of metals from their ores using carbon eg iron from iron oxide Metals low down in the activity series (like copper and lead) can be used for roofs. Metals even lower (silver and gold) are used for jewellery and electrical contacts. How metals react with water, acids and other metal oxides Reactive metals react more vigorously in air or with acids (releasing hydrogen) The burning splint test for hydrogen Reactive metals can replace a lower metal from its oxide 11 The Earth and limestone Limestone is made from calcium carbonate Useful as a building material. Easily cut but weathers easily Limestone reacts with hydrochloric acid to form carbon dioxide gas. Carbon dioxide dissolves in rain water making the rain acidic which attacks the limestone. When limestone is heated it decomposes forming carbon dioxide and leaving agricultural lime (calcium oxide) Acid rain. 12 Materials from the ground Extraction, purification and uses for aluminium, iron and copper 2 Physics Energy forces and space 1 Measurement Measurement of and units for distance, mass and time. The period of a pendulum as an example of measurement Measurement of density Density = mass ÷ volume unit of density = kg/m3 or g/cm3 Calculate density of solid objects (regular and irregular shape) (use displacement of water to find volume of irregular solid) Calculate density of liquids That air has mass and we can calculate its density Floating and sinking. 2 Force Unit for force. Using a spring balance. Measurement, advantages and disadvantages of friction (Air resistance, streamlining, stopping distances as in highway code) Magnetism, magnetic field lines, poles, attraction and repulsion between poles. Springs in series and parallel. Drawing graph plotting extension against load. Levers: use of levers to change direction and magnitude of a force and their use in simple machines, e.g. crowbars, pliers, Measurement of speed. Speed = distance ÷ time. Units for speed (m/s). Mass, weight and gravity. 3 Pressure Moments about a pivot; that the unit of a moment is a newton metre (or newton centimetre) Relationship between force, pressure and area. centimetre) Pressure = force ÷ area. Units for pressure. (N/cm2) Application of pressure [e.g. the use of skis and snowboards, the effect of sharp blades] 4 5 Light Reflection. Angle of incidence = angle of reflection Light changes direction when it meets a boundary Refraction . Dispersion. How a prism disperses white light similar to a rainbow. and sound Comparing speed of light and sound Amplitude volume. Frequency and pitch. Energy Law of Conservation of Energy. Energy forms. Energy conversions. Generating electricity Energy loss from buildings. Energy from sun. Wind and waves. Water cycle 6 Particle theory All matter made of particles which are always moving. Using kinetic theory to explain solids liquid, gasses, evaporation and freezing, expansion and contraction 7 Electricity Measurement of current through lamps. Resistance. Using an ammeter to measure current. Series and parallel circuits. Switches, (reed, SPST, push) LED's, LDR's, relays. AND and OR circuits (made using switches) and truth tables 3 8 The Earth in space The universe, galaxy and The solar system. Eclipse of sun and moon Seasons. Day and night. Satellites: moon and artificial. Phases of the moon 4 Biology Organisms, their behaviour and environment 1 Cells Name and function of main structures. that a typical animal or plant cell has a nucleus, cytoplasm, mitochondria and cell surface membrane Nucleus contains genetic material which contains DNA Difference between animal and plant cells. Plant cells contain a vacuole All plant cells have a cell wall and Some plant cells contain chloroplasts Asexual reproduction: budding Using a microscope. Preparing A microscope slide using a stain like methylene blue Cells form tissues which form organs Main organs in a human: their function and name 2 Classification Features of main animal and plant groups. Using a simple key. 3 Life processes The brain and nervous system. Response to a stimulus Energy made available through aerobic respiration. Know the equation for respration Test exhaled air using limewater to show it contains carbon dioxide use of energy for warmth, movement, growth, cell repair and various chemical processes Digestion occurs when enzymes in the gut, like amylase, break down food into soluble substances that can be absorbed into the bloodstream across the villi in the small intestine. Waste products are egested through the anus and excretion Diet: Carbohydrate, protein, fats, vitamins, minerals, fibre water Blood circulation.: artery, vein, capillaries, heart and lungs 4 Flowering plants Structure. Leaves, stem, flower and roots. Roots have root hairs to increase surface area Pollination, fertilization and fruit formation, structure and dispersal of seed, germination. Photosynthesis. Word equation Formation of glucose which is converted to starch. Starch test Importance of photosynthesis in providing food and oxygen How to do a controlled experiment to show that light is needed for starch production. The starch can be tested for using iodine solution 5 Microbes and health Nutrition. Nitrates are needed for healthy growth and magnesium for the production of chlorophyll. Viruses, bacteria and fungi Bacterial disease (eg cholera) viral disease eg Flu. Uses in industry and nature. Fermentation of yeast. Importance of cleanliness and hygiene (wash hands, keep cooking surfaces clean etc) The importance of exercise and healthy eating;( Low fat, low salt) keeps the heart healthy, improves stamina, makes you feel better. 5 6 Human life cycle The structure and mechanism of the human reproductive system The terms embryo, foetus, gamete and zygote Fallopian tube, urethra, uterus, ovary, vagina, prostate gland, testis, penis Fertilization occurs when sperm and egg fuse together The relative size and numbers of the egg and sperm and how they are brought together. The role of the egg and sperm. How the joining of egg and sperm bring genes from both parents together which gives the baby the characteristics of both parents. Development of embryo and role of placenta. How the foetus is protected and nourished in the uterus and how its waste materials are eliminated 7 Ecology Changes at puberty. Menstrual cycle Physical and emotional changes at adolescence How to study of a habitat: measurement of animal or plant populations Using a quadrat Study of an animal and plant. Energy flow along food chain. Food webs. Comparison between sexual and asexual reproduction How to measure a physical factor in the environment like temperature or light intensity. How population size is effected by predation or competition The importance of conserving habitats How to use a key to identify an animal or plant using its features Environmental and inherited causes of variation Discontinuous and continuous variation The carbon cycle and its role in maintaining a balance between photosynthesis and respiration 6 Investigations 1 Method Know how to carry out a CONTROLLED experiment (Carry out two experiments, changing one and not the other [the control]) Understand how to carry out a fair test and realise the importance of a fair test. (so the results can be compared with accuracy). Tests should be repeated and the average found: this makes the results more reliable Change only one variable (the independent variable) and measure the dependent variables. Keep all other variables the same) 2 Apparatus Know the names on apparatus used to measure. Metre rule, stopwatch, spring balance, top pan balance, bourdon gauge, measuring cylinder, beaker Know the units on apparatus used to take measurements 3 Recording results Know the names and use of apparatus used in chemistry and biology Correct use of tables and graphs Labelling graphs with correct units Using the correct type of graph (line or bar graph) 4 Some example investigations Showing how effective an insulation is at keeping water warm. Showing that light intensity controls the rate of photosynthesis. Showing how the resistance of a wire increases with length Showing how the solubility of a solvent increases with temperature Showing the presence of starch in a leaf,. Measuring the speed of a ball rolling down a slope 2 SCHEME OF ASSESSMENT 13+ Assessment of the 13+ syllabus can occur at two levels: Level 1 and Level 2. The syllabus is common for both levels, although those parts of the syllabus which are underlined will only be assessed on Level 2 papers. It is envisaged that candidates who are expected to achieve less than an average of 40% on the three Level 2 papers should consider using the Level 1 paper. Level 1 (80 marks; 60 minutes) There will be one paper with approximately equal numbers of questions based on the 13+ biology, chemistry and physics syllabuses. The paper will consist of a mixture of closed items, e.g. multiple choice, matching pairs, completing sentences and some open questions. Open questions will have several parts, some of which will require answers of one or two sentences. These parts will carry a maximum of two marks. Up to 10% of the marks on the paper will be available for plotting graphs or making simple calculations, such as calculating means from data or using a formula. There will be no choice of questions. The use of calculators and protractors will be allowed in the examination. Level 2 (60 marks per paper; 40 minutes per paper) There will be three papers, one in each of biology, chemistry and physics. Some of the questions may be closed, although most will be open with several parts requiring candidates to answer in sentences. These parts will carry a maximum of three marks. In addition, one mark may be given for an acceptable standard of spelling, punctuation and grammar in one part of the paper. The maximum number of marks per question will be twelve. At least 25% of the paper will be testing how science works. There will be no choice of questions. The use of calculators and protractors will be allowed in the examination. SCHOLARSHIP Scholarship papers are based on this syllabus. The Common Academic Scholarship examination (90 minutes, including 10 minutes’ reading time) will be divided into three sections: A (Biology), B (Chemistry) and C (Physics). Each section will contain two questions. Candidates will be required to attempt three questions, one from each section. Each question will carry 20 marks. The use of calculators and protractors will be allowed in the examination. 3