Graphing Reaction Rates: Chemistry Assignment
Name ______________________ Date________________ Due Date _______________
Graphing Reaction Rate Assignment
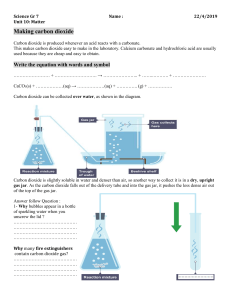
Solid calcium carbonate will react with a solution of hydrochloric acid to produce carbon dioxide gas according to the following equation:
HCl(aq) + CaCO
3
(s) H
2
O(l) + CO
2
(g)
The gas is collected in a gas measuring tube, and the volume of carbon dioxide gas collected is recorded at the time intervals below:
Elapsed Time (s)
0.0
2.0
4.0
6.0
8.0
Volume of CO
2
(ml)
0.0
10.0
17.0
22.0
26.0
10.0
12.0
14.0
29.0
30.0
31.0
16.0
18.0
31.5
31.5 a) Use graph paper to plot the volume of carbon dioxide gas released (y axis) as a function of time (xaxis). Be sure to label the x and y axis appropriately and include units. Give the graph an appropriate title. b) Find the instantaneous rate of production of carbon dioxide gas in ml/s at the point where 2s has elapsed. c) Find the rate of reaction when 12s has elapsed. d) Explain, with reference to the concentrations of reactants, why the answers to parts b) and c) are different. e) Assuming that the reaction takes a total of 16 s to reach completion, what is the average rate of production of carbon dioxide gas measured in ml/s. (change in volume/change in time) f) Explain how would the graph plotted in part a) have differed if the temperature had been raised?