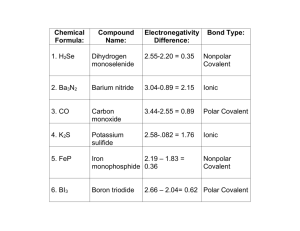
Name_______________________ Answers to Final Exam Study
advertisement

Name_______________________ Answers to Final Exam Study Guide I. Matter and Changes 1. Iron (III) Nitrate is completely dissolved in water to make a transparent (can see through) blue solution. This solution may be classified as: A) pure substance C) element B) heterogeneous mixture D) homogeneous mixture 2. Hydrochloric acid actively reacting with metals to create hydrogen gas is an example of a: A) chemical property C) physical property B) chemical change D) physical change 3. Which of the following is not an example of a physical change? A) water boiling C) lead melting B) sugar dissolving D) food souring 4. Which of these steps in the digestive process is a physical change? A) Saliva changing carbohydrates to sugars B) The hormone insulin metabolizing C) Enzymes breading down proteins to amino acids D) Liquids being absorbed in the large intestine 5. Bones are made up of many minerals, one of which is Calcium. What can calcium be classified as? A) homogeneous mixture C) element B) compound D) heterogeneous mixture 6. Which state of matter is characterized by high density and low compressibility? A) gas C) liquid B) solid D) all of the above 7. Ms. Bailey had a glass of water and tacos with lettuce, cheese, and chicken for lunch. Which of the following correctly classifies what she ate? A) Water: Compound B) Water: Compound Tacos: Heterogenous Mixture Tacos: Homogenous Mixture C) Water- Element D) Water: Element Tacos: Compound Tacos: Heterogenous Mixture 8. Which of the following is an extensive property of a bubble of H2 gas? A) percent oxygen C) density B) radius D) temperature 1 Name_______________________ Answers to Final Exam Study Guide II. Atomic Structure, Matter and Nuclear Chemistry 9. Consider an element Ub that has two naturally occurring isotopes with the following percent abundances: the isotope with a mass number of 11.0 is 42.0% abundant; the isotope with a mass number of 18.0 is 58.0% abundant. What is the average atomic mass for element Z? (Answer with 2 decimal places.) a. 15.10 g c. 15.35 g b. 15.06 g d. 15.92 g 10. Which model supports Rutherford’s discovery of the nucleus? a. The nucleus of made of neutrons only. b. The nucleus is made of protons, neutrons and electrons. c. The nucleus is positive and outside the nucleus is negative. d. The nucleus is made of electrons and protons. 11. In a fusion reaction, what is happening to the atom involved? a. Energy is absorbed. c.The nucleus is combined with another nucleus. b. Energy does not change. d.The nucleus is split. 12. What particle is needed to complete this nuclear reaction? Mn + H ____ + 5 Co a. c. 51 26 Fe b. Fe d. Cr 13. Which of the following types of radiation is the most difficult to protect yourself from? a. b. Gamma Beta c. d. Alpha All of the above 14. What particle is needed to complete this equation? N a. + ______ C + He 4 1 2 Name_______________________ a. n Answers to Final Exam Study Guide b e e c. d. 15. Nuclear fission ________. a. Produces hydrogen nuclei. b. The separation of one nucleus to form two nuclei of smaller mass c. Occurs when large nuclei fuse together. d. All of the above. 16. Which of the following types of radiation has negligible mass and does not change the number of protons or neutrons in an atom’s nucleus? a. Alpha c. Beta b. Gamma d. None of the above III. Data Analysis and Nuclear Chemistry 17. Round the following measurement to three significant figures: 0.50971 cm2. A) 0.51 cm2 C) 0.509 cm2 2 B) 0.510 cm D) 0.5097 cm2 18. How many significant figures are in the measurement 102.410 meters? A) five C) four B) six D) three 19. Convert 28,500 waves/sec into scientific notation. A) 2.85x104 waves/sec C) 2.85 x103 waves/sec -3 B) 2.85x10 waves/sec D) 2.85x10-4 waves/sec 20. Perform this calculation: 4.6x10-5 divided by 2.5x10-3 A) 0.018 C) 0.152 B) 1.6 D) 6.58 21. Which nuclear particle has the least penetrating power and lowest mass? This Question is mistaken. Alpha has the least penetrating power, and beta has the lowest mass out of the options. A) a positron ( ) C) a neutron ( ) B) a beta particle ( 22. Beta particles A) are helium nuclei ) D) an alpha particle ( ) C) can be stopped by lead 3 Name_______________________ B) Answers to Final Exam Study Guide consist of two protons and four neutrons D) all of the above 23. The equation below shows the radioactive decay of thorium (Th). ? Which of the following particles is released in this reaction? A) proton B) beta C) neutron D) alpha 24. The element Hydrogen has 3 naturally occuring isotopes. The isotope with a mass of 1.00 is 98.5% abundant; the isotope with a mass of 2.00 is 1.00% abundant; and the isotope with a mass of 3.00 is 0.500% abundant. What is the average atomic mass of Hydrogen? None of the answer choices are correct for this problem. The answer is 1.02. A) 1.008 amu C) 1.155 amu B) 1.21 amu D) 1.342 amu IV. Electromagnetic Spectrum and Electron Configuration 25. What is the electron configuration for this element? 2p 2s 1s a. b. 1s12s22p6 1s22s22p2 c. d. 1s22s22p4 1s11p12p6 26. How many valence electrons are present in the element with an atomic number of 16? a. b. 1 6 c. d. 5 7 27. 4 Name_______________________ Answers to Final Exam Study Guide Which label on the model represents the amplitude and wavelength respectively? a. b. Q, S R, T c. d. S, Q T, R 28. UV and IR light are invisible to the human eye. What makes IR light less dangerous than UV light? a. IR light has longer wavelenths, and higher energy b. IR light has shorter wavelengths, and lower energy c. IR light has shorter wavelengths, and lower energy d. IR light has longer wavelengths, and lower energy 29. What is the frequency of a wave that has a wavelength of 5x10-7m? a. b. 3.33 x10-14 Hz 6.00 Hz c. d. 6 x1014 Hz 2.00x10-17 Hz 30. Which element is represented by the following electron configuration? 1s22s22p63s23p64s1 a. b. Sc Ar c. d. K Ca 31. What block is represented by the number 2? a. b. s d c. d. f p 32. How many valence electrons are shown in this picture? a. b. 8 3 c. d. 5 11 5 Name_______________________ Answers to Final Exam Study Guide 33. Name this element: 2p 2s 1s a. b. C N c. d. O F 34. What is the lowest energy state of an atom? a. Ground State b. Hund’s Rule c. Excited State d. Aufbau Principle 35. Which of the following states that an electron occupies the lowest available energy orbital? a. Ground State b. Hund’s Rule c. Excited State d. Aufbau Principle V. Periodic Table and Trends 36. Of the following elements Ga, Ar, Xe, and I, which is a metal? a. b. Xe c. Ar I d. Ga 37. A mystery element Q can be cut with a knife easily, and will produce hydrogen (H 2) gas when put into water. The reaction is very explosive. What group is this element most likely found in? a. Group 18, Noble Gas c. Group 17, Halogens b. Group 2, Alkaline earth metals d. Group 1, Alkali metals 38. What underlining similarities explain how elements are arranged in rows and columns on the periodic table? a. similarity in size b. similarity in melting point c. d. similarity in symbol similarity in properties 39. When moving across a period (from left to right), what happens to electronegativity, and the atomic radius? a. Electronegativity decreases and Atomic Radius increases 6 Name_______________________ b. c. d. Answers to Final Exam Study Guide Electronegativity increases and Atomic Radius decreases Electronegativity increases, and Atomic Radius increases. Electronegativity decreases, and Atomic Radius increases. 40. Based on the following elements which group correctly shows increasing ionization energy? a. Be, Mg, Ca, Sr, Ba b. F, Cl, Br, I, At c. d. Al, Si, P, O, F F, O, B, C, N 41. Which model below appropriately identifies the Noble Gases? a. c. b. d. 42. What is electronegativity? a. one half the distance between nuclei of identical atoms b. c. d. the distance from the nucleus to the outer orbital of an ion tendency of an atom to attract electrons in a chemical bond energy required to remove an electron from an atom VI. Bonding and Nomenclature 43. Which type of bond is found in sodium iodide? a. b. covalent ionic c. d. hydrogen metallic 44. In which of the following is the name and formula given correctly? a. b. Sodium nitride, NaN Aluminum Sulfate, Al2S3 c. d. cobaltous fluoride, CoF3 tin (IV) fluoride, SnF4 45. Given the ionic compound CuF, what is the name of this compound? a. b. copper (I) fluoride copper (II) fluoride c. d. copper (I) fluorate copper (II) fluorate 46. Which of these elements exist as a diatomic molecule? 7 Name_______________________ a. b. Li B c. d. Answers to Final Exam Study Guide Cl C 47. What type of geometry does BCl3 have? a. b. pyramidal c. trigonal planar d. bent tetrahedral 48. Hexane (C3H6) and Calcium Chloride (CaCl2) have very different boiling points. Hexane boils at 68 °C, while Calcium Chloride melts at 1,935 °C. What is the best explaination for the difference in boiling points? a. Hexane is polar and Calcium Choloride is an ionic compound. c. Hexane is a covalent compound and Calcium Chloride is an ionic compound. b. Hexane is an ionic compound and Calcium Chloride is a covalent compound. d. Both are ionic, the reason for the difference is unknown. 49. A mystery compound X is insoluble (does not dissolve) in water, and compound Y does. Compound X and Y are most likely a. covalent, covalent c. ionic, ionic b. covalent, ionic d. ionic, covalent 50. What is the formula of trinitrogen pentafluoride? a. b. N2F5 N3F5 c. d. NF3 N5F10 8