review packet

1. Which set of pictures illustrate an isotope & an ion of the same element:
2. Which atomic symbol represents an isotope of sulfur with 17 neutrons? a) 17 X b) 33 X c) 17 X d) 49 X
16 16 32 32
3) Write out the electron configurations for elements
#21 - 36
4. Which is the electronic configuration of calcium? a) 1s 2 2s 2 2p 6 3s 2 3p 8 b) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 c) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 d) 1s 2 2s 2 2p 8 3s 2 3p 6
5. On this picture of the Bohr atomic model, draw arrows and labels to show a) the capture of energy by an e- & what
happens to the e-. b) the result of an e- moving back to a lower
energy level & where spectral lines come from.
6. An electron in an atom of hydrogen goes from energy level 6 to energy level 2. What is the wavelength of the electromagnetic radiation emitted? a) 410 nm b) 434 nm c) 486 nm d) 656 nm
*7. On the chart given, draw out a hypothetical redioactive decay
*8.The half-life of a radioactive isotope is 20 min. What is the total amount of 1.00 g of sample of this isotope remaining after 1 hour? a) 0.500g b) 0.333g c) 0.250g d) 0.125g
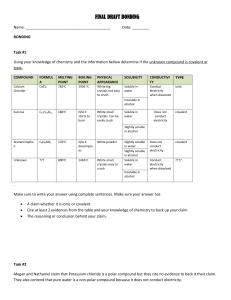
+9. Put the following bond types in order from strongest to weakest: covalent, ionic, metallic
+10. Which statement correctly compares the amount of energy needed to break the bonds in CaCl
2
(E1) and
C
6
H
12
O
11
(E2)? a) E1>E2 as CaCl2 is a covalent compound b) E1<E2 as CaCl2 is a covalent compound c) E1>E2 as CaCl2 is an ionic compound d) E1<E2 as CaCl2 is an ionic compound
+11. For each pair of atoms, decide if it will make an ionic or a covalent compound, then create the correct formula. hint: if no charge is given, find what it is! atoms_______predict bond________formula____
Rb + F
C -4 + H
S -2 + O
Fe +3 + SO
4
12. Which statement describes the compound formed between sodium & oxygen? a) NaO
2
& it's ionic b) NaO
2
& it's covalent c) Na
2
O & it's ionic d) Na
2
O & it's covalent
+13. The 3 inter-molecular forces are: Dipole-dipole interactions, London Dispersion (aka Van der Waals) forces & hydrogen bonding. Put these forces in order of weakest to strongest. Explain why a substance with the weakest inter-molecular force would evaporate fastest
& the substance with the strongest force would evaporate slowest.
+14. At STP, fluorine is a gas and iodine is a solid. Why? a)Fluorine has lower average kinetic energy than iodine b)Fluorine has higher average kinetic energy than iodine c)Fluorine has weaker intermolecular forces of attraction than iodine. d) Fluorine has a stronger intermolecular forces of attraction than iodine.
15. Write the formulas for each of the following named compounds:
Copper II Chloride
Sodium Phosphate
Di-hydrogen monoxide
Nitrogen tri-sulfide
16.What is the IUPAC name for the compound represented by the formula Ca(OH)
2
? a) Calcium hydroxide b) Calcium di-hydroxide c) Calcium II hydroxide d) Calcium II di-hydroxide
+17. An unknown substance is tested in the laboratory.
The physical test results are listed below:
nonconductor of electricity
insoluble in water
soluble in oil
low melting point
Based on these results, what is the unknown substance? a) ionic & polar c) covalent & polar b) ionic & non-polar d) covalent & nonpolar
18. List 3 elements for each P.Table group. Include the element's group & period.
Group______________Element____Row / Period alkali metals alkali earth
metals halogens
Noble gases transition metals any metals any non-metal any metalloid
19. An atom has 8 protons & 9 neutrons. Which statement best describes the element? a) It is a non-metal in Grp. 2 b) It is a non-metal in Grp. 16 c) It is a metal in Grp. 2 d) It is a non-metal in Grp 17
20. What will likely be some properties of Element 117 when it is discovered?
21. Which atom has the largest radius? Justify your answer. a) Bromine b) Chlorine c) Selenium d) Sulfur
22. Arrange the following elements in order of increasing electronegativity, from lowest to highest.
They are: F, K, Si & S
23. What causes the process of perspiration to be cooling for the human skin? a) It involves condensation and is exothermic b) It involves evaporation and is exothermic c) it involves condensation and is endothermic d) it involves evaporation and is endothermic
*24. Explain when boiling occurs using the terms 'vapor pressure' & 'atmospheric pressure'
25. Given the heating curve below, what is occuring between minutes 6 to 12? a)There is an increase in kinetic energy & vaporization is occurring. b) There is an increase in kinetic energy & condensation is occrring. c) There is an increase in potential energy and freezing is occurring. d) There is an increase in potential energy and melting is occurring.
26.According to the phase diagram below, what is the boiling point of this substance at 1.25 atm?
27. An 8.80 g sample of metal is heated to 92.0
0 C and then added to 15.0 g of water a 20 o C in an insulated calorimeter (Styrofoam cup) At thermal equilibrium (all heat has flowed and everything is the same temp.) the temperature of the system was measured as 25.0
0 C.
What was the identity of the metal? m1(
T1)Cp = m2(
T2)Cp
(metal) (water)
T1 is X
28. 1000 J of heat is added to 2 g of the following substances. Which one will experience the biggest change in temperature? a) aluminum b) copper c) iron d) lead
29. Compare the Cp's for each element in question 28.
Which one would have the smallest change in temperature under the conditions of #28. Why?
30. What causes an inflated balloon to shrink when it is cooled? a) because cooling the balloon causes gas to escape from the balloon. b) because cooling the balloon causes the gas molecules to collide more frequently. c) because cooling the balloon causes gas molecules to become smaller. d) because cooling the balloon causes the average kinetic energy of the gas particles to decrease.
31. If the Kelvin temperature and the pressure of (not on) a sample of gas are doubled, what will be the effect on the density of the gas? Draw labeled pictures that explain your answer.
32. In the energy diagram below, which statement describes the forward reaction? a) It is an exothermic reaction with an energy change of
160 kJ. b) It is an exothermic reaction with an energy change of
80 kJ. c) It is an endothermic reaction with an energy change of 160 kJ. d) It is an endothermic reaction with an energy change of 80 kJ.
33.For the following energy curve, answer the following: a)Label the energy of the reactants & products, the activation energy, the enthalpy of the reaction and where the activated complex is. b)exothermic or endothermic reaction? c)explain how a catalyst can speed up a reaction.
34.A student mixes two chemicals in a test tube. The test tube turns hot and bubbles appear. What indicators of chemical reaction is the student observing? a) Change in color and formation of precipitate b)Change in color and formation of gas.
c) Change in temp. & formation of a ppt. d) Change in temp. & formation of a gas.
35. Select the indicators of a chemical reaction that would help you distinguish between these two reactions.
1. Sodium metal dropped into a beaker of water.
2. Silver Nitrate solution is added to sodium chloride dissolved in water. a) Write a balanced chemical equation for each reaction
(including phases as subscripts).
1.
2. b) Identify each type of reaction.
1.
2.
36. Balance the following combustion reaction equation:
C
4
H
10
+ O
2
--> CO
2
+ H
2
O
37. 10.3 grams of sodium hydrogen carbonate reacts with an excess of hydrochloric acid. A gas is made and after evaporation, a white crystalline substance is produced and its mass is 7.59 g. a) What type of acid reaction occurred? b) Write the balanced reaction equation. c) What is the identity of the white crystal? d) Figure out how many moles of sodium hydrogen carbonate were used.
38. Given the balanced chemical reaction equation:
P
4
+ 5O
2
--> P
4
O
10
What mass of oxygen is needed to completely react with 7.75 g of P
4
? hint: mass-mass (aka 3-step) prob.
39. 70 g of limestone (CaCO
3
) was reacted with HCl and
14.0 L of CO
2 gas is made. What % of the limestone
(CaCO
3
) is CO
2
? a) Figure out how many moles of the limestone you have (that's the whole} b)Figure out how many moles of the CO
2
you have (this is the part) c) figure out the %.
40.A compound consisting of 56.3% phosphorus and
43.62% oxygen has a molecular mass of 220 g/mol.
What is the molecular formula? a) Figure out the EMPIRICAL Formula as you've been taught. b) Find the molar mass of the EMPIRICAL Formula. c) Divide that molar mass by 220. Multiply each empirical subscript by the answer for your true, molecular formula.
41. A 10.10 g sample of barium chloride hydrate is heated in a crucible. After all of the water is evaporated off, 8.50 g of the anhydrous barium chloride remains in the crucible. What is the formula of the hydrate? a) Remember the original amount is the whole. Figure out the % of water that left and the % of barium chloride that remained. b) Use the two %s to work an empirical formula.
42. List the 5 factors that affect a reaction rate.
43. When a set amount of marble rock (CaCO
3
) is added to Hydrochloric acid, a reaction occurs. What should be done to decrease the rate of the reaction the next time the experiment is performed? a) Use more acid b) Stir c) Use larger marble chips d) Add heat.
44.Draw and label a picture that explains when a system is at equilibrium:
45. A scientist observes a chemical reaction as i takes place. What can the scientist do in order to tell if the reaction has achieved equilibrium? a) Measure the concentrations of products and
reactants over time. b) Monitor the temperature of the reaction over time. c) Measure the pH of the solution over time. d) Wait for the formation of a precipitate.
46.For the reaction
2SO
2
(g) + O
2
(g) <==> 2SO
3
(g) + heat a) write out an action that will increase the [SO
3
] b) write out an action that will decrease the [SO
3
]
47. Based on hydroxide concentration , which unknown substance would be an acid? a) Substance A [OH -1 ] = 1 X 10 -2 M b) Substance A [OH -1 ] = 1 X 10 -4 M c) Substance A [OH -1 ] = 1 X 10 -6 M d) Substance A [OH -1 ] = 1 X 10 -8 M
48.Given the data below, which substance is the acid?
49.What volume of 0.20M HCl will neutralize 10 mL of
0.40 M KOH?
50. If 25.0 mL of 12.0 H
2
SO
4
is diluted to a total volume of 1 L (1000 mL) what is the new concentration? hint: it's still M1V1 = M2V2!
51. Heating a solution does what? a) increases the solubility of a solid solute. b) increases the solubility of a gas solute c) increases the miscibility of the solution. d) increases the degree of saturation of the solution.
52. According to the solubility graph:
How many grams of KCl are required to make a saturated solution in 50.0 g of water at 80 0 C? a) 25 g b) 50 g c) 100 g d) 150 g
53.When considering the thermodynamics (heat exchange) of the solution process, which is ALWAYS considered exothermic? a) Solute particles separate from one another. b) Solvent particles separate from one another. c) Solute and solvent particles form attractions for one another. c) Any solution formation is ALWAYS exothermic.
54.Explain why the solubility of NH
3
gas decreases in water as the solution temperature rises.