Answer Key Review Exam 1 1.Atom 2. Compound 3. Molecule 4
advertisement

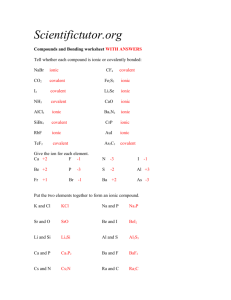
Answer Key Review Exam 1 1.Atom 2. Compound 3. Molecule 4.Mixture 5. Nucleus is the protons and neutrons Neutons(0) determine isotope and mass Protons (+) determine element Electron(-) determine charge Group 1 Alkali Metals Group 2 Alkaline Earth Metals Group 17 Halogens Groups 18 Noble Gases Metals are on the left of the periodic table Semimetals are located on a staircase in between the nonmetals and metals Nonmetals are in the upper left Matching 1. 2. 3. 4. 5. 6. Bc Df Ad Eb Fe Ca tera T 1 terameter (Tm) = 1012 m giga G 1 gigameter (Gm) = 10s m mega M 1 megameter (Mm) = 106 m kilo K 1 kilometer (Km) = 103 m deci d 1 decimeter (dm) = 10-1 m centi c 1 centimeter (cm) = 10-2 m milli m 1 millimeter (mm) = 10-3 m nano n 1 namometer (nm) = 10-9 m angstrom Å 1 angstrom (Å) = 10-10 m Sig Figs 1. 10.68 2. 72.98 3. 29 4. 1.80 5. 2.3 Rounding/Scientific notation 1.6.599X10^1 2. 7.84x10^-4 3. 3.9X10^4 4.6.7834X10^-5 5. 9X10^2 Temp Conversions 1. 21.95 C 2. 170 F 3. 19.4 C = 290 K 4. Boiling 373K 212F Freezing 273K 32F DA 1. a. 76mL b. 50nm c. 1.55g/L d. 6.151X10^-3 e.4.32X10^5 f. 707.9 cm^3 2. 2.52 kg air P or C 1. Chemical 2. Physical 3. Physical 4. Chemical 5. Chemical Laws 1. Mass is neither created nor destroyed. 2. A pure chemical substance always contains the same ratio of elements 3. If 2 elements A and B combine to from more than one compound than the mass of B that can combine with A is a small whole number. 4. 5.00g D, Conservation of mass Chart in class activity Ion-cation, loses,anion, gains Isotopes B Average atomic weight= 24.31amu Naming/Ionic/Covalent 1. Magnesium oxide, ionic, 2 2. Sulfur hexafluoride, covalent,0 3. Coppe(II) sulfide,ionic, don’t worry about it 4. Dinitrogen tetroxide,covalent,0 Compound 1. CaCl2,ionic, 3 2. P4S10,covalent,0 3. Cu(ClO)2,ionic,don’t worry about it 4. Al(NO3)3 Balancing Equations 1. 4,3,2 2. 2,7,4,6 3. 2,1,2 4. 2,3,1,3, 6 mol CuO Formula mass and molar mass are the same number but FM is in amu and MM is in g/mol Formula Weight a. 342.0amu b. 164.1amu Conversions 1. 6.022X10^23 molecules of H2O 2. 0.874 mol C, 5.26X10/////623 3. 0.08267 mol MgCl2