File - gpat victory
advertisement

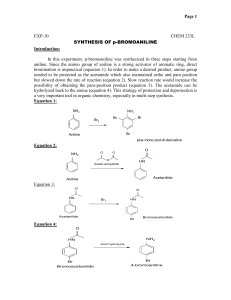
Date: Pg.No: PRACTICAL : 01 TITLE:- Synthesis of p-nitro aniline. AIM(i):- To synthesize p-nitro acetanilide from acetanilide. REFERENCE:- Online edition for students of organic chemistry lab courses at the University of Colorado, Boulder, Dept of Chem and Biochem. (2004), REQUIREMENTS:APPARATUS:Conical flask(250ml,100ml ),Measuring cylinder(100ml), beaker(500ml), pipette(10ml),Condenser, Round bottom flask(100ml),Buchners funnel, filter paper. REAGENTS:Acetanilide , Glacial Acetic Acid, Ethanol, Nitric Acid, Sulfuric acid,NaOH. PRINCIPLE: Step 1: In this step we first form the nitronium ion in situ by dehydration of nitric acid. Sulfuric acid is the dehydrating agent. The nitronium ion is a very powerful Date: Pg.No: electrophile and will react with the π electrons of the aromatic ring of aniline. These reactions are shown in Figure. Step 2: Through deprotection of acetamido functional group of p-nitro acetanilide into amino. It is a Simple acid catalysed hydrolysis reaction. REACTION MECHANISM:- PROCEDURE:- It includes following two steps:- Date: Pg.No: 1) Synthesis of p-nitro acetanilide from acetanilide and 2) Preparation of p-nitro aniline from p-nitro acetanilide. CHEMICAL INGREDIENTS FOR STEP 1: COMPOUND Acetanilide G.Acetic acid Ethanol Nitric acid Sulfuric acid Mol wt(gm/ mol) 135.17 60.05 46.07 63.01 98.079 Quantity Taken B.P./M.P. ( °C) 115 118-119 78 83 337 Density (gm/cm 3 ) 1.16 1.049 0.8 1.5129 1.84 Hazards Irritant,toxic Corrosive flammable Caustic Caustic Synthesis of p-nitro acetanilide from acetanilide. 1. Take 3.4 gm of Acetanilide. 2. Dissolve in 4ml glacial actetic acid, in a small conical flask. 3. Take 5ml of conc.H2SO4, when all material is dissolved, cool in ice bath, crystals may begin to form. 4. Add 5ml of ice cold H2SO4 dropwise. 5. Cool the solution upto 5°C. 6. In separate conical flask take 1.8ml of conc.HNO3 and 2.5ml of H2SO4. 7. Cool this mixture of HNO3 and H2SO4, and add this solution dropwise to above mixture with stirring. 8. Keep the solution to stand at room temperature for 30 mins. 9. Pour in ice cold water (50ml). 10. Collect the product by sucktion with buckners funnel, and wash with water and dry. 11. Recrystallize from 95% Ethanol. Date: Pg.No: Aim(ii):- To synthesize p-nitro aniline from p-nitro acetanilide. CHEMICAL INGREDIENTS FOR STEP 2: COMPOUND Mol wt Hazards p-nitro acetanilide NaOH Quantity B.P./M.P. Density (gm/mol) Taken ( °C) (gm/cm3) 180.16 215 1.42 irritant 39.997 Caustic 318 M.P. 2.13 Preparation of p-nitro aniline from p-nitro acetanilide. 1. 2. 3. 4. 5. Transfer the crude p-nitro acetanilide in 100ml round bottom flask. Add 10ml water and 10ml conc.HCl. Reflux the mixture for 20 mins. Then cool it on room temperature and add 15ml of cold water. Then add either 10% NaOH solution or Ammonia solution, until the solution becomes basic. 6. Collect the precipitates by filtration, wash with water and dry it. 7. Recrystallize the product by boiling water. PREPARATION OF TLC PLATE:Set aside a small, spatula tip of the crude product in a small test tube. We will use this in the TLC analysis step. Take silica gel in beaker and add adequate water to form slurry. Now transfer this slurry on a glass slide and speard evenly on the surface, and dry it in air. Date: Pg.No: Now take n-hexane and ethyl acetate as a mobile phase in 4:1 ratio in a beaker. For activating stationary phase ,keep the prepared plate in oven at 120°C for 30 mins. Perform the spotting by following method: Mark four small dots with your pencil evenly spaced along the baseline of a fresh TLC plate. Spot 1 -pure p-nitroaniline (Obtain this from the stockroom.) Spot 2 -crude p-nitroaniline Spot 3 -recrystallized p-nitroaniline Spot 4- pure o-nitroaniline (Obtain this from the stockroom.) Now put this slide in the beaker containing the mobile phase . And after the mobile phase has ran to the level required take the slide out from beaker and measure its Rf value . Rf value = Distance travelled by solute Distance travelled by solvent RESULT :Theoritical yield Practical yield Percentage yield Melting point Rf value