PreLab
advertisement

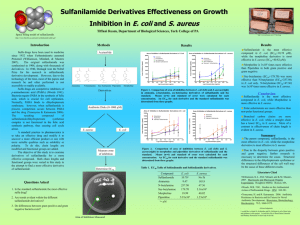
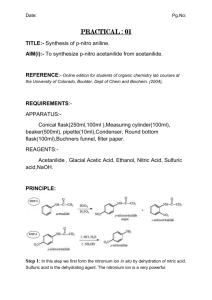
Katie Masters Desk #17A November 13, 2003 TA: Karen Heichel Experiment: The Synthesis of Sulfanilamide, An Antibacterial Drug Chem 36 Section 1 PreLab Summary Sulfanilamide, or p-aminobenzenesulfonamide, was first synthesized in 1908. It was discovered that sulfanilamide has antibacterial properties, and the mode of action has been determined. Bacteria must synthesize folic acid in order to grow. Sulfanilamide inhibits the formation of folic acid, and therefore, stops the growth of bacteria. Since humans do not synthesize folic acid (must be acquired in food), only bacteria are affected by the use of sulfanilamide. Thus, there is no negative side effect to taking sulfanilamide since it does not disrupt any critical biological action in the body. This experiment focuses on the three-step synthesis of sulfanilamide, starting with acetanilide. The synthetic scheme is shown in the “Diagrams of Special Apparatus/Reaction” section of this PreLab. In this three-step route, acetanilide is subjected to chlorosulfonation, which is then followed by sulfonamide formation, and finally, reduction of the acetyl amide to yield sulfanilamide. The final product, sulfanilamide, will be crystallized and weighed to determine the percent yield of the pure product. The melting point and IR spectrum will be acquired to verify the purity and identity of sulfanilamide. Reference: Handout downloaded from the Chem 36 website, www.courses.chem.psu.edu/chem36. Goals To synthesize sulfanilamide via a three-step process starting with acetanilide. To purify sulfanilamide via recrystallization. To achieve a 90% recovery (as reported in the experimental procedure) for the recrystallization of the crude product. To verify sulfanilamide’s purity and identity via melting point and IR analysis. Diagrams of Special Apparatus/Reaction Scheme Hydrogen Chloride Trap Apparatus: Reaction Scheme of the Synthesis of Sulfanilamide: H N O C H N O C CH3 CH3 NH3 + HSO3Cl SO2Cl Acetanilide H N O C chlorosulfonic acid CH3 p-acetaminobenzenesulfonyl chloride NH2 1. HCl (aq) SO2NH2 p-acetaminobenzenesulfonamide 2. NaHCO3 SO2NH2 sulfanilamide Experiment Chemical Data Table Chemical Name Structure* Physical State BP or MP Liquid Density Quantity MW FW Mmol Flammability Toxicity Price Waste Comment Acetanilide Solid 113113°C (MP) N.A. 0.25 g 135.17 1.85 No Yes $17 for 100 g NHO Irritant Chlorosulfonic acid Liquid 1.753 g/mL 0.625 mL 116.52 9.40 No Yes $61 for 1 L D Corrosive; reacts violently with water pAcetaminosulfonyl chloride Solid 151152°C at 755 mm Hg (BP) 145148°C (MP) N.A. 223.67 No No $18 for 100 g HO Corrosive pAcetaminobenzene sulfonamide** Solid Sulfanilamide Solid 165167°C (MP) N.A. 172.21 No No $46 for 50 g NHO Irritant *Note: Structures are not shown in this table (due to limitations in computer application program), but are required in all Chemical Data Tables. **Not found in Aldrich or online at ChemFinder.com Note: Although NH3 and NaHCO3 are reagents used in this synthetic route, they are not included in the Chemical Data Table. These compounds are listed on the Common Chemical Data Table and need not be listed here. Chromatographic Behavior Comparison of Starting Material & Product Acetanilide vs. Sulfanilamide Sulfanilamide will have a lower Rf due to the more polar NH2 group and sulfonamide group (SO2NH2) - two polar groups. Acetanilide only has one polar amide group, thus will have a higher Rf value. Spectral Features Comparison of Starting Material & Product 1H NMR, Main Differences: Acetanilide will have 5 signals in its NMR spectrum while sulfanilamide will have 4 signals. The aromatic protons for sulfanilamide will be shifted more downfield (in the range of 7 to 8 ppm) due to the presence of more de-shielding groups. These aromatic protons will appear as two doublets. Acetanilide’s aromatic protons will appear as a triplet and two doublets. IR, Main Differences: Acetanilide will have an amide carbonyl stretch at ~1680 cm-1. Sulfanilamide will have, mostly likely, two N-H stretches for the primary amine group at ~3300-3500 cm-1 and S=O stretch at ~1340cm-1. MS, Main Differences: Acetanilide will show a M+ peak at 135; sulfanilamide will show a M+ peak at 172. Acetanilide may show a –CH3 (-15) fragment where sulfanilamide will not. Explanation of Isolation (Work-Up) and Purification Sulfanilamide: Once the reaction is complete, a yellow solution will remain that will be shaken with granulated decolorizing charcoal, which will be filtered by removal of the solution with a pipette. The charcoal will serve to remove the colored impurities from the solution. The resulting colorless solution will be mixed with an aqueous solution of sodium bicarbonate in order to neutralize the hydrochloride. The pH will be tested with indicator paper to ensure that the solution is neutral. The suspension will be cooled in ice to precipitate out the sulfanilamide product. The solid will be collected via vacuum filtration (isolation of product). The crude product will be recrystallized from water to purify sulfanilamide. PreLab Exercise Draw out the mechanism for the reaction of p-acetaminobenzene sulfonamide to form sulfanilamide. H+ H SO2NHR O SO2NHR O N H N H H O H H+ H H O H SO2NHR O H H O N H O N H H SO2NHR O H3C SO2NHR OH + H2N