Laboratory 4
advertisement

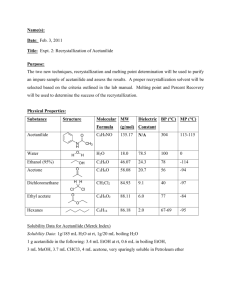
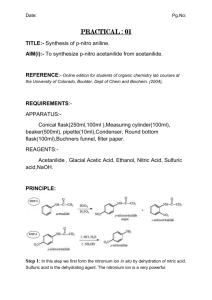
EXPERIMENT Purification of Acetanilide by Recrystallization 3 Prepared by Verrill M. Norwood, Cleveland State Community College OBJECTIVE Students will separate and purify acetanilide from a mixture by recrystallization. They will then compare the melting points of impure and recrystallized acetanilide and calculate percent recovery. Impurities often contaminate organic compounds that have been synthesized in the laboratory or isolated from natural sources. Recrystallization is a purification technique used to remove impurities from organic compounds that are solid at room temperature. This technique is based on the knowledge that the solubility of a compound in a solvent increases with temperature. Since the solubility of a compound decreases as the solution cools, crystals form. The choice of solvent is crucial in purification by recrystallization. In this experiment, impure acetanilide is dissolved in just enough boiling water to form a saturated solution. Then a five percent excess of water is added to the saturated solution to prevent premature crystallization. For example, if 20 mL of boiling water is required to just dissolve the acetanilide, five percent of 20 mL or 1 mL would be added to bring the total volume to 21 mL. Often, a sample will contain an insoluble impurity. In that case, the hot solution is filtered by gravity filtration through a funnel containing a fluted filter paper to remove the insoluble compounds. After the acetanilide is dissolved in a minimal amount of boiling water and the saturated solution is filtered, the solution is allowed to slowly cool to room temperature. If crystal formation occurs too rapidly, impurities and some solvent may become trapped in the crystals. Very small crystals are undesirable because it is difficult to wash them free of the soluble impurities, and they take longer to dry. Usually, the best compromise of speed, convenience, and quality of crystals is obtained by allowing the solution to cool to room temperature on a non-heat conducting surface such as a cork ring. The filtered solution can then be cooled in an ice-water bath for a few minutes to maximize crystal formation. Vacuum filtration is the best method for separating the crystals from the mother liquor, or remaining solvent. In vacuum filtration, a receiver flask with a sidearm, called a filter flask, is connected by heavy-walled vacuum tubing to a vacuum source. A Büchner funnel is fitted to the filter flask with a rubber stopper or filter adaptor. The most common source of the vacuum in vacuum filtration is a water aspirator. To recover the purified acetanilide, the Büchner funnel plate is covered with a filter paper disk, which is moistened with water. With vacuum applied, the solution containing the recrystallized acetanilide is poured onto the filter paper so that a uniform thickness of crystals collects in the middle the paper. After the mother liquor has been pulled through the filter, the crystals are washed with small portions of ice-cold water. The crystals are then dried and their mass is measured. Percent recovery is calculated by dividing the mass of the recrystallized acetanilide by the mass of the impure compound before recrystallization, and multiplying the result by 100. The purity of the recrystallized acetanilide is assessed by measuring its melting point range. The melting point of pure acetanilide is reported as 114oC. A pure compound melts over a narrow range of 1-3 oC near its reported melting point. Directions: WORK IN PAIRS Caution: Wear departmentally-approved safety goggles at all times while in the chemistry laboratory. 1. Weigh about 2 g of impure acetanilide [Data Sheet] and place it into a 100-mL beaker (label as Beaker 1). 2. Place approximately 60 mL of distilled water [Data Sheet] into a 100-mL beaker (label as Beaker 2). Add a boiling stone. Using a hot plate, heat the water to boiling (start out on a high temperature and once the solution boils, reduce the temperature as necessary to maintain a slow boil). 3. Using beaker tongs, pick up Beaker 2. Use a disposable pipet to add approximately 2.0 mL portions of boiling water to the beaker containing the acetanilide. Stir the contents of Beaker 1 using a stirring rod with each addition of boiling water. Keep the water in both beakers at boiling by placing them on the hot plate as necessary. Continue adding the boiling water in 2.0 mL portions until the acetanilide just dissolves. 4. Using beaker tongs, remove the beakers from the hot plate. Allow the acetanilide solution in Beaker 1 to cool below the solvent (water) boiling point (100oC). Observe and record the solution color [Data Sheet]. 5. Measure and record [Data Sheet] the volume of water remaining in Beaker 2. Calculate the additional solvent volume needed to have a 5% excess of water in Beaker 1 (measured volume * 0.05). Use a 10-mL graduated cylinder to measure and add the additional solvent volume to Beaker 1. 6. Assemble a gravity filtration apparatus. Your completed apparatus will consist of two parts: (1) a stemless funnel with a piece of fluted filter paper in it (fold the filter paper in half and then in half again so that a cone shape is formed, unfold, lap over one fold section and mold to inside of funnel), and (2) a 250-mL beaker. Conduct the procedures in Steps 7, 8, and 9 simultaneously [Note 1]. NOTE 1: So that crystals will not form in the funnel, plan to filter the boiling solution from Step 9 using the filter apparatus from Step 8 while the filter apparatus is still hot. 7. Place 50 mL of water into a 100-mL beaker. Add a boiling chip. Heat the water to boiling on the hot plate. 8. Using beaker tongs, pick up the hot beaker containing the boiling water. Preheat the gravity filtration apparatus by pouring the boiling water through the stemless funnel containing the fluted filter paper. Do not allow the boiling chip to go into the funnel. Collect the 50 mL of water in the 250-mL beaker. Place the gravity filtration apparatus on the hot plate to keep the water hot. 9. Reheat the saturated acetanilide solution in Beaker 1 to boiling. 10. Using beaker tongs, place the stemless funnel on to another clean, dry 250-mL beaker, being careful not to puncture the filter paper. Pour the hot solution from Step 9 into the stemless funnel and filter the solution. 11. Allow the hot, saturated solution of acetanilide from Step 10 to cool to room temperature. When the solution has reached room temperature, place the 250-mL beaker into an ice-water bath for 5 minutes. Be careful that your beaker does not tip over in the ice-water bath!!! 12. While the solution is cooling in the ice-water bath, assemble a vacuum filtration apparatus in the hood, using a 500-mL vacuum filter flask (see example in Instructor’s hood). Also, prepare a washing solvent by placing 5 mL of distilled water into a test tube. Cool the test tube and its contents in the ice-water bath. 13. Weigh a piece of filter paper and record its mass [Data Sheet]. Once crystallization is complete, turn on the vacuum, and moisten the filter paper with a few drops of water. Swirl the 250-mL beaker containing the purified acetanilide, and pour the crystals and mother liquor into the Büchner funnel, using a glass rod to direct the crystals to the middle of the filter paper. 14. After the mother liquor has been pulled into the filter flask, turn off the vacuum and release the vacuum by carefully disconnecting the vacuum hose from the side arm of the filter flask. Remove the Büchner funnel from the filter flask and pour the mother liquor into a beaker. Reattach the Büchner funnel and vacuum hose, and turn the vacuum back on. 15. Use 2-4 mL portions of the mother liquor to rinse the remaining crystals of acetanilide from the 250-mL beaker. Pour the rinses into the Büchner funnel. 16. Wash the crystals in the Büchner funnel with 1-2 mL portions of the ice-cold water (Step 12). Allow the crystals to dry by pulling air through the funnel for 10 minutes. Disconnect the vacuum tubing and turn off the vacuum. Carefully remove the filter paper and crystals, and place it on a watch glass. Place the watch glass on top of a warm drying oven. 17. During the next available class period, come back to the lab and weigh the filter paper and recrystallized acetanilide [Data Sheet]. 18. Measure and record the melting point ranges of the impure and recrystallized acetanilide [Data Sheet]. 19. Place your recrystallized acetanilide into a labeled vial and turn it in to your instructor. Waste Disposal Any aqueous solutions generated in this lab can be flushed down the sink with plenty of water. Lab Report: Once you have turned in your Instructor Data Sheet, lab attendance will be entered and you will be permitted to access the online data / calculation submission part of the lab report (click on Lab 3 – Purification of Acetanilide by Recrystallization). Enter your data accurately to avoid penalty. The lab program will take you in order to each calculation. If there is an error, you will be given additional submissions (the number and penalty to be determined by your instructor) to correct your calculation. Post-Lab Questions: Any post-lab questions for this lab can be found at http://www.Chem21Labs.com. Do Not Wait Until The Last Minute To Do These!!!! Laboratory 3 Purification of Acetanilide by Recrystallization Student Data Sheet Mass of Impure Acetanilide (~ 2 g) Melting Point of Impure Acetanilide g oC Recrystallization: Volume of Distilled Water in Beaker 2 (~ 60 mL) mL Color of Acetanilide Solution Volume of Distilled Water Remaining in Beaker 2 mL Recovery: Mass of Filter Paper g Mass of Filter Paper + Acetanilide g Melting Point of Purified Acetanilide oC Laboratory 3 Purification of Acetanilide by Recrystallization Instructor Data Sheet Mass of Impure Acetanilide (~ 2 g) g Recrystallization: Volume of Distilled Water in Beaker 2 (~ 60 mL) mL Color of Acetanilide Solution Volume of Distilled Water Remaining in Beaker 2 mL Recovery: Mass of Filter Paper g