Acetanilide Synthesis: Lab Report & Recrystallization

Emily Kirschenbaum
Chem 203 Section 2
TA: Kristin Beiswenger
4/10/14
Introduction
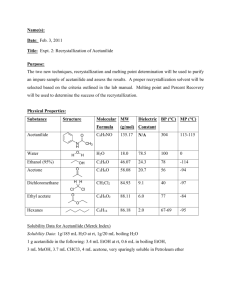
The purpose of this experiment was to synthesize acetanilide in a reaction between aniline and acetic anhydride and evaluate recrystallization as a purification technique. In order to recrystallize acetanilide, a suitable solvent was chosen based on the concept that “likes dissolve likes”. Acetanilide is an important medicinal compound because of its pain-relieving abilities. It prevents pain by blocking the pulse dispersing throughout the nerve fiber (Binoy, et al, 2006).
To verify this product was obtained, melting point analysis of both crude and recrystallized products, NMR and IR data were used to confirm the identity and purity information for the acetanilide. By calculating the percent yield and recovery, the efficiency of the synthesis was determined. Recrystallization was a good technique for isolating very pure compounds by easily removing impurities that stay behind in the solvent. This method worked best because only particles of the compound were able to join the lattice structure of the crystallizing compound.
Reaction Mechanism
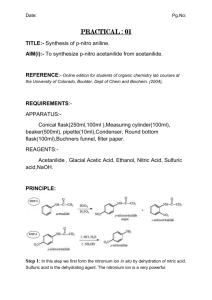
Aniline is the nucleophile and the carbonyl of acetic anhydride is the electrophile. The aniline nucleophile attacks the acetic anhydride electrophile via nucleophilic addition, breaking the double bond.
The negatively charged nucleophile oxygen gives up its electrons to form a double bond.
Simultaneously, the bond breaks between the carbon and oxygen, separating into two compounds via nucleophilic elimination.
The negatively charged oxygen takes a proton from the positively charged nitrogen via deprotonation. The nitrogen and oxygen become neutrally charged, and the desired product acetanilide has been formed.
Recrystallization is a purification method that obtains pure crystals from a supersaturated solution by leaving all impurities in the solvent. The impure solid is mixed with hot solvent until mostly dissolved. As the solution cools, the pure crystals form without any impurities mixed in when performed correctly. Recrystallization is most effective when there are small amounts of impurities present in the solid product, and this is the case with acetanilide. In order for the process to work, a solvent with similar polarity to the product must be selected. The best solvent will cause the product to dissolve at high temperatures and have poor solubility at low temperatures, because recrystallization occurs when the solid precipitates out of solution as it cools and leaves the impurities within the solvent solution (Bortiatynski, 2013-2014).
The chosen methods to confirm identity and purity of the product are NMR, IR, and melting point determination. NMR is a useful tool to obtain structural information and IR depicts which specific functional groups are present in the product. Both methods will reveal any impurities in the product. Determining the melting point of the solid will show whether the product is likely to be acetanilide, but does not confirm identity. Only spectroscopic analysis using NMR and IR will absolutely confirm the structure in order to be certain the acetanilide product was obtained in its purest form.
Experimental Section
2
Synthesis of Acetanilide: 2mL of aniline and 15mL of deionized water were stirred and 3mL of acetic anhydride was added drop wise. The mixture was stirred for about 30 minutes. Vacuum filtration was used to isolate the product, which was then rinsed with ice water, and dried.
Finding a Suitable Recrystallization Solvent: Four solvents (water, ethanol, dichloromethane, and hexanes) were tested for solubility with the product obtained. The solubility was tested at room, boiling, and freezing temperatures.
Purification of Acetanilide: The chosen solvent was heated before adding to the solid. The minimum amount of hot solvent was added to the solid to dissolve the product until clear. Once clear, the flask was removed from the heat and allowed to slowly come to room temperature.
Then an ice bath was used to cool the crystals until the temperature was consistent with the ice water. Vacuum filtration was used to collect the crystals and they were thoroughly rinsed with ice water, and then dried.
Data
Melting Points:
Crude melting point = 100º - 105ºC
Recrystallized melting point = 110º - 113ºC
Percent Yield of Crude Acetanilide: 92% yield
Percent Yield of Pure Acetanilide: 81% yield
Percent Recovery of Acetanilide from the Reaction: 90%
3
Table 1: NMR for Acetanilide Table 2: IR for Acetanilide
NMR Peaks
Observed
Peak
Specific
Hydrogen
2.2ppm CH
3
(methyl)
7.0-7.6ppm H (aromatic)
7.8ppm NH
This table represents the NMR data, which gives structural information about the product.
Significant Signals
Bond Stretch Observed
1657.0 cm
-1
3059.5 cm
-1
3288.8 cm -1
C=O
C-H (aromatic)
R-NH-R’
This table represents the IR data, which gives information about the functional groups present in the product.
Table 3: Solvent Solubility
Solvent
H
2
O
Ethanol
Dichloromethane
No heat Heat Cooled
Chunky, slightly soluble Mostly soluble Solid precipitates out
Completely dissolves ------------------ ---------------------------------
Completely dissolves ------------------ ---------------------------------
Hexanes Chunky, mostly insoluble Mostly soluble Not much precipitation out
This table shows the data obtained from solvent solubility analysis that was used to choose an appropriate solvent for recrystallization.
Results and Discussion
After performing the reaction, the product obtained was analyzed using various techniques. Before beginning this analysis, the physical appearance of the crude product was noted as being a white solid that appeared crystalline. The melting point of this crude product was 100º-105ºC. The expected melting point for acetanilide is 112º-116ºC. Since the crude product had not been purified, this depression in melting point makes sense. A depression in
4
melting point is caused by impurities being trapped in the liquid phase of the solid. This makes the liquid phase more favorable, thus causing a lower melting point (Hock, et al, 2008). To purify the product, a suitable solvent was chosen using the criteria that it should dissolve the product at high temperatures and precipitate out at low temperatures. Water, ethanol, dichloromethane, and hexanes were the solvents chosen to test for solubility with the product.
The data in table 3 gives evidence for ethanol and dichloromethane being ruled out because they dissolved the product immediately without any heat. When added to hexanes, the product was mostly insoluble without heat, mostly soluble in heat, but not much product precipitated out. The product was slightly soluble in water without heat, completely soluble when heated, and then as it cooled the product precipitated out. Acetanilide, the product, is a polar compound because of the amide group. Using the concept that “likes dissolve likes”, water is a good solvent because it is also a very polar molecule. Ethanol and dichloromethane are medium polarity solvents; this made them good contenders for this recrystallization, but they showed complete solubility without heat which ruled them out. The fact that hexanes are very nonpolar made it an unlikely solvent choice because the product would not dissolve well in it, even at higher temperatures.
Water had similar polarity with the product, but the slight difference allowed for a solid to precipitate out. Water fit the recrystallization solvent criteria very well and was chosen as the solvent.
With water as the recrystallization solvent, the crude product was purified. White crystals were obtained from the recrystallization and the melting point of this pure product was 110º-
113ºC. This melting point fit well with the expected acetanilide melting point of 112º-116ºC, but does not confirm the identity of the product. To confirm the identity of the product, NMR and IR spectroscopy were used. According to table 1, the NMR showed significant peaks at 2.2 ppm,
5
7.0-7.6 ppm, and 7.8 ppm. The peak at 2.2 ppm corresponds to the hydrogens of the methyl group. Methyl has three hydrogens which contribute to the integral value and it is isolated from the other hydrogens in acetanilide, which gives it a singlet splitting pattern. The peaks from 7.0 to 7.6 ppm represent the aromatic hydrogens. Aromatic hydrogens are very similar and interact with one another, which causes the multiplet splitting pattern in this region of the spectra. The small peak at 7.8 ppm corresponds to the hydrogen on the nitrogen. These peaks match up very well with the structure of acetanilide. There are no other peaks present on the NMR spectrum, which confirms identity and purity of the acetanilide product. If the product was not formed from the reaction, the aniline starting material would show a significant peak between 3 and 5ppm.
This peak is not present, which means any leftover aniline starting material was purified out during recrystallization. IR spectroscopy was used to verify the results from the NMR spectra.
According to table 2, there are three significant signals at 1657.0 cm -1 , 3059.5 cm -1 , and 3288.8 cm
-1
. The signal at 1657.0 cm
-1
corresponds to the carbonyl stretch, 3059.5 cm
-1
to the methyl carbon-hydrogen bonds, and the 3288.8 cm
-1 to the nitrogen-hydrogen group. These signals further verify that acetanilide is the product obtained from the synthesis reaction and it is also pure in its composition.
To determine the efficiency of recrystallization as a purification technique, the percent recovery was determined. Percent recovery of acetanilide from the purification was 90%. The percent yield was used to determine how much of the desired product was obtained when compared to the expected theoretical yield from the molar ratios of the reaction. The crude product yield was 92% and the pure product yield was 81%. The reason the crude product yield is much higher than the yield of the pure product is because there was water present in the crude
6
product when the mass was taken and also impurities that had yet to be removed from the recrystallization.
The reaction itself was simple, because it went all the way to completion by simply stirring it for 30 minutes. Vacuum filtration was also a very simple and effective method to obtain the desired solid. The most difficult part was isolating the solid, because particles would travel at the slightest breeze. To minimize this problem, working quickly to put the product in a safe container was an effective solution.
Conclusions
The synthesis of acetanilide using aniline and acetic anhydride starting materials was successful. This was evident by the melting point determination, NMR, and IR spectroscopy.
These three methods of identification together clearly demonstrated that acetanilide was obtained from the synthesis reaction. The efficiency of recrystallization as a purification technique was evident by the 90% recovery of acetanilide. The efficiency of the synthesis reaction was evident by the 81% pure product yield after purification. Water, the chosen solvent, was the correct choice because the results matched up with expectations. The product was somewhat soluble without heat, dissolved when heat was added, and then precipitated out as a crystalline solid as the solvent cooled slowly. In the future, it would be interesting to investigate the effects of cooling rate during recrystallization on the purity of the product obtained. This would allow for a more thorough understanding of the mechanism behind recrystallization.
References
Binoy, J., Prathima, N.B., Krishna, C.M., Santhosh, C., Joe, I.H, Jayakumar, V.S. (2006).
Influence of intermolecular amide hydrogen bonding on the geometry, atomic charges,
7
and spectral modes of acetanilide: an ab initio study, Laser Physics , 16(8), pp. 1253-
1263.
Bortiatynski, J. Lab Guide for Chemistry 203, 2013-2014, pp. 141 – 167.
Hock, C., Strassburg, S., Haberland, H., Issendorff, B.V., Aguado, A., Schmidt, M. (2008).
Melting-point depression by insoluble impurities: A finite size effect, Physical Review
Letters , 101(2).
8