Name(s): Date: Feb. 3, 2011 Title: Expt. 2: Recrystallization of
advertisement

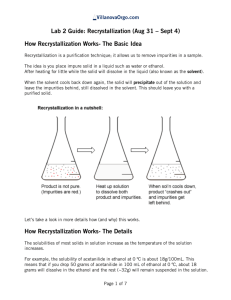
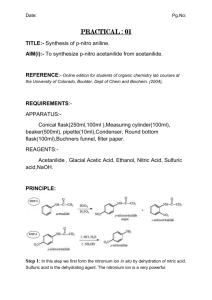
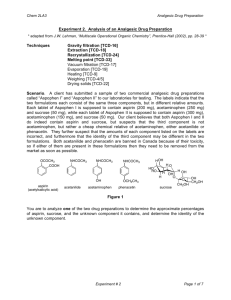
Name(s): Date: Feb. 3, 2011 Title: Expt. 2: Recrystallization of Acetanilide Purpose: The two new techniques, recrystallization and melting point determination will be used to purify an impure sample of acetanilide and assess the results. A proper recrystallization solvent will be selected based on the criteria outlined in the lab manual. Melting point and Percent Recovery will be used to determine the success of the recrystallization. Physical Properties: Substance Structure (g/mol) Constant C8H9NO 135.17 N/A 304 113-115 H2O 18.0 78.5 100 0 C2H6O 46.07 24.3 78 -114 O C3H6O 58.08 20.7 56 -94 H H CH2Cl2 84.93 9.1 40 -97 C4H8O2 88.11 6.0 77 -84 C6H14 86.18 2.0 67-69 -95 O H Ethanol (95%) Ethyl acetate N H O CH3 H OH Acetone Dichloromethane Dielectric BP (°C) MP (°C) Formula Acetanilide Water Molecular MW Cl O Cl O Hexanes Solubility Data for Acetanilide (Merck Index) Solubility Data: 1g/185 mL H2O at rt, 1g/20 mL boiling H2O 1 g acetanilide in the following: 3.4 mL EtOH at rt, 0.6 mL in boiling EtOH, 3 mL MeOH, 3.7 mL CHCl3, 4 mL acetone, very sparingly soluble in Petroleum ether References: 1. CHM 235 Spring 2011 Lab Manual from Dr. Halligan titled “Experiment 2: Recrystallization of Acetanilide,” pp. 16-26. 2. Sigma Aldrich Online Catalog: http://www.sigmaaldrich.com/ Diagrams: Figure 2.2: Vacuum filtration using a Buchner Funnel Figure 2.2 Mel-Temp apparatus Experimental Procedure: 1. Prepare a melting point capillary tube for crude acetanilide and set aside. 2. Obtain six test tubes and place approximately 20 mg of crude acetanilide in each. 3. Add the following six solvents (1 mL each): 95% ethanol, acetone, dichloromethane, ethyl acetate, hexane and water were added to the test tubes. 4. Record result of dissolution at room temperature for each sample after swirling. 5. Heat those that did not dissolve in the solvent at room temperature in a boiling water bath for a few minutes. 6. Record dissolution of the solid when heated for each sample. 7. Place the heated test tubes in an ice bath and note those that crystallize. 8. Verify a suitable recrystallization solvent with the instructor. 9. Mass the remaining crude acetanilide and place into a clean 125-mL Erlenmeyer flask. 10. Recrystallize this sample with water. 11. Use decolorizing charcoal to remove the color impurities. 12. After heating with charcoal for a few minutes, add two spatula tipfuls of celite to aid in the removal of the charcoal. 13. Filter the hot mixture through fluted filter paper to retain the charcoal and the celite. 14. Cool the filtrate to room temperature slowly. 15. Clamp the sample in an ice bath to complete crystallization. 16. Collect the pure acetanilide on a Buchner funnel by vacuum filtration. 17. Rinse the filter cake (crystals) with two small portions of ice-cold water and dry. 18. Note appearance of the recrystallized acetanilide. 19. Record the mass of the recrystallized product. 20. Calculate Percent Recovery. 21. Obtain the melting point of the recrystallized and crude acetanilide products.\ Calculations: observed mass of pure acetanilide Percent Reocvery = X 100% initial mass of impure acetanilide Data Checklist: Solvent Dissolved at rt Dissolved w/ heat Crystallized w/ ice Water Ethanol (95%) Acetone Dichloromethane Ethyl acetate Hexanes Recrystallized product appearance: Recrystallized Product mass: Crude acetanilide melting point: Recrystallized acetanilide melting point: Post-Lab Questions: 1. Why is it important to warm the fluted filter paper and Erlenmeyer flask with hot solvent before filtering the dissolved acetanilide solution? 2. How successful was your purification of acetanilide? What data helps support your conclusion and how? 3. How does an impurity affect the melting point of a solid?