Nitration - Synthesis of p
advertisement

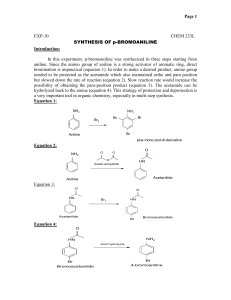
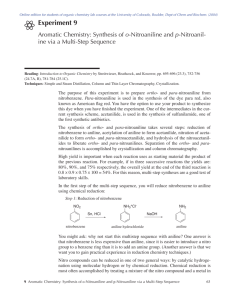
CH421 Experiment 2 Nitration of Aniline: a three-step protecting group strategy to prepare p-nitroaniline Reading: Organic Chemistry by John McMurry, 8e, Chapter 16.1-16.5 Techniques: recrystallization, melting point, IR, NMR Introduction Electrophilic aromatic substitution is one of the more common ways to functionalize aromatic rings, and one common method is nitration. The reactivity of the aromatic ring is heavily influenced by substituent effects, specifically resonance and/or inductive effects of the substituent. The purpose of this experiment is to prepare para-nitroaniline from aniline. Para-nitroaniline is used in the synthesis of the dye para red (aka American flag red). One of the intermediates in our synthetic scheme, acetanilide, was also used in the synthesis of sulfanilamide, one of the first synthetic antibiotics. We will perform the synthesis of para-nitroaniline over several steps (overall scheme shown below): acetylation of aniline to form acetanilide; nitration of acetanilide, which forms a mixture of ortho- (minor) and para-nitroacetanilide (major); and hydrolysis of the nitroacetanilides to liberate ortho- and para-nitroanilines. Separation of the ortho- and para-nitroanilines is accomplished by recrystallization. When performing multi-step synthesis, high yield is important, since each reaction uses as starting material the product of the previous reaction. For example, if in three successive reactions the yields are: 80%, 90%, and 75% respectively, the overall yield at the end of the third reaction is 0.8 x 0.9 x 0.75 x 100 = 54%. For this reason, multi-step syntheses are a good test of laboratory skills. In the first step of the sequence above, aniline is acetylated with acetic anhydride to produce acetanilide. Note that the desired final product in this experiment is a mono-nitroaniline. Why, then, is the step of acetylating the aniline included in the reaction scheme? Essentially, the amide is used as a protecting group for the aniline in this scheme (keep in mind amines can act as bases and nucleophiles; amides in contrast are non-basic, non-nucleophilic). First, aniline can be oxidized by nitric acid. (If aniline and nitric acid are mixed directly, a violent oxidation of the aniline occurs which can cause the mixture to ignite.) In addition, under these acidic reaction conditions, aniline is protonated, and the anilinium cation is actually a meta-director! Thus, in order to successfully and selectively mononitrate aniline at the para position, it is necessary to modify the amine as the amide, perform the nitration reaction, and remove the acetamide protecting group at the end of the synthesis the liberate the amine. The second step in our sequence is the nitration of acetanilide. Nitronium ion (NO2+) is a very strong electrophile that is generated in situ from concentrated nitric acid and sulfuric acid, as shown below. Experiment 2: Nitration: Synthesis of p-Nitroaniline In the mechanism of this reaction, the nucleophilic pi electrons on the aromatic ring attack the electrophile NO2+. The resulting intermediate carbocation is short-lived, since aromaticity is lost upon addition of NO2+. Elimination of the paraproton restores aromaticity, resulting in an overall substitution reaction. In electrophilic aromatic substitutions where there is an electron-donating group (EDG, such as –OMe) on the aromatic ring, the electrophile substitutes in the ortho- and/or para- positions relative to the EDG. This is due to resonance stabilization of the intermediate carbocation by the EDG. On the other hand, if there is an electron-withdrawing group (EWG) on the aromatic ring, reaction is directed to the meta position. In this reaction, the electrophile substitutes mainly at the position para to the acetanilide, since the amide is an electron-donating group (EDG) by resonance. While we want to obtain the p-nitroacetanilide selectively, in reality we obtain a mixture of isomers, wherein pnitroacetanilide is the major isomer (see scheme below). Acetanilide displays moderate reactivity in electrophilic aromatic substitution as it is a weak EDG. Also, unlike aniline, acetanilide is not oxidized by nitric acid. Nitration of acetanilide gives principally the ortho and para mononitroacetanilides (with more para than ortho due to sterics), together with a small amount of 2,4-dinitroacetanilide. To prevent dinitration of the acetanilide, the nitrating mixture of concentrated nitric acid and sulfuric acids is added in small portions to the acetanilide solution (and not vice versa), so that the concentration of the nitrating agent is kept at a minimum. The nitroacetanilides are not isolated in this reaction scheme. Instead, the reaction mixture is heated and the acid present serves to hydrolyze them to the nitroanilines (see scheme below). Separation of the para- and ortho-nitroanilines is effected by recrystallization in ethanol due to the different solubility of each compound in ethanol. In Week 1, we will prepare acetanilide (Step 1), isolate it, and fully characterize the crude product. In Week 2, we will perform two steps: nitration of acetanilide (Step 2), followed by hydrolysis of the acetamide (Step 3) to generate the desired nitroaniline product. Experiment 2: Nitration: Synthesis of p-Nitroaniline Safety Precautions Nitrobenzene, aniline, and nitroaniline are toxic; aniline is also a cancer suspect agent. Sulfuric acid, nitric acid, acetic anhydride, and ammonium hydroxide are extremely caustic; avoid skin contact. If contact does occur, flush with water for 15 minutes. Wear your gloves and protective clothing throughout this experiment and work in the fume hood to avoid breathing vapors. Week 1: Preparation of Acetanilide Pre-Lab Notebook (to be completed prior to the start of lab in Week 1) • • Enter date, experiment number and title on notebook page and in TOC with corresponding page number. Copy the Week 1 reaction and the reaction table below (in your own writing) into your notebook and complete all empty cells in the table. Make sure to check your calculations with your instructor before starting the experiment. MW (g/mol) Name formula mass (g) mmol equiv aniline C6H7N 1.50 1.00 acetic anhydride C4H6O3 _____ 1.20 water H2O d (g/mL) vol (mL) mp (°C) ___ ____ bp (°C) ___ desired rxn conc. ____ 1.0 ____ ____ 0.27 M Lit. mp acetanilide C8H9NO ____ ___ Week 1 Procedure Into a 125-mL Erlenmeyer flask, weigh out 1.50 g of aniline (NOTE: always record the actual mass that you measured to the precision of your balance), add a stir bar, and dissolve this in enough water to give a concentration of 0.27 M (you should record the VOLUME of water used). Add 1.2 equivalents of acetic anhydride, and stir the solution at room temperature for at least 20–30 minutes. At some point, solids should form. Note any changes that occur to the reaction mixture and the times at which they occurred. After 20-30 minutes, cool the reaction mixture in an ice bath to complete crystallization. Isolate the product by vacuum filtration, wash it with cold water, and allow it to dry on the funnel by pulling air through the solids for 20 minutes. It is imperative that your acetanilide be dry for the next step! Thus, label a small beaker, obtain a tare weight, and put your produce in it covered with a Kimwipe/rubber band to dry in the back of the hood for a week. Experiment 2: Nitration: Synthesis of p-Nitroaniline Data for Week 1 (collect and record in your notebook during the lab) • • • • • Describe product and calculate crude % yield of the dried solid Obtain melting point and list with literature value Obtain IR (solid is best), staple in notebook, and assign major functional group peaks Obtain 1H NMR (use ~10 mg in CDCl3), staple into notebook, and assign all peaks in the spectrum Record beaker tare weights Week 2: a) Nitration of Acetanilide; b) Hydrolysis of Nitroacetanilides Pre-Lab Notebook (to be completed prior to the start of lab in Week 2) • • Enter date, experiment number and title on notebook page and in TOC with corresponding page number. Copy the Week 2 reaction and the reaction table below (in your own writing) into your notebook and complete all empty cells in the table. Make sure to check your calculations with your instructor before starting the experiment. O HN NO2 p-nitroaniline acetanilide MW (g/mol) NH2 1. H2SO4, HNO3 2. H2O, heat 3. NH4OH mass (g) mmol equiv d (g/mL) vol (mL) mp (°C) bp (°C) Name formula acetanilide C8H9NO 0.900 1.00 conc. H2SO4 H2SO4 _____ 8.0 ___ ___ conc. HNO3 HNO3 _____ 2.0 ___ 140 conc. NH4OH NH4OH _____ 33.9 ____ ___ p-nitroaniline C6H6N2O2 ___ ___ Lit. mp ____ ____ ____ ___ Experiment 2: Nitration: Synthesis of p-Nitroaniline Week 2 Procedure Measure 8 equivalents of concentrated sulfuric acid into a 10-mL graduated cylinder (this volume should be in your notebook, as you calculated it previously). Pour about half of this acid into a 50-mL round bottom flask. To the acid remaining in the graduated cylinder, add 2.0 equivalents of concentrated nitric acid (again, record volume). Carefully mix the two acids in the cylinder by filling a Pasteur pipet at the bottom of the cylinder, and then discharging it at the top, several times. Add 0.90 g of your dry acetanilide from Week 1 to the sulfuric acid in the Erlenmeyer flask, swirl until almost all of it has dissolved, and then cool the flask in an ice bath. Using a Pasteur pipet, transfer about ¼ mL portions of the nitric-sulfuric acid mixture to the flask and swirl the flask in the ice bath after each addition. Do not allow the flask to become warm; the addition of all of the mixture of acids should require about 10 min. When the addition of acids is complete, swirl for 2 minutes, then add to the reaction mixture 10 mL of ice water and a boiling chip. Attach a water-cooled condenser to the flask. Using a heating mantle as the heat source (Variac setting ~ 70), gently boil the mixture for 15 min to effect hydrolysis of the nitroacetanilides. Cool the flask in an ice bath. When the reaction mixture is thoroughly cold (make sure it and the ice bath are directly under your hood), CAUTIOUSLY add a total of 9 mL of concentrated ammonium hydroxide in approximately 1 mL portions, taking care to swirl the flask after each addition. Make sure that the mouth of the flask is not pointed at yourself or your neighbor. This neutralization reaction is quite exothermic, and if done improperly, it will result in a sudden evolution of ammonia that expels the contents out of the flask! Collect the crude product by vacuum filtration, wash the product in the Büchner funnel with a total of 4 mL of cold water, and dry it by drawing air through it for 5-10 minutes. Run a TLC of your crude product in 1:1 hexanes:ethyl acetate, draw the plate in the Data section and calculate Rf’s for all spots. The crude product contains both ortho- and para-nitroaniline. As stated in the introduction to this experiment, the major isomer is para-nitroaniline. (The amount of dinitroaniline present is negligible.) Recrystallize the crude product from about 2 mL of hot ethanol in a 25-mL Erlenmeyer flask. Allow the mixture to slowcool to room temperature, and then set it in an ice bath for 10-15 min. Isolate the resulting solids by vacuum filtration use 1-2 mL of cold ethanol to transfer remaining crystals to the funnel and to rinse the crystals on the filter paper. Dry the solids by pulling air through the funnel for 10 minutes. Run TLC of your purified product in 1:1 hexanes:ethyl acetate, draw the plate and calculate Rf in your Data section. Data for Week 2 (collect and record in your notebook during the lab) • Describe product and calculate % yield of the recrystallized solid • TLC data (draw crude and purified product TLC’s, calculate Rf’s, include mobile phase) • Obtain melting point and list with literature value • Obtain IR (solid is best), staple in notebook, and assign major functional group peaks • Obtain 1H NMR (use ~10 mg in CDCl3), staple into notebook, and assign all peaks in the spectrum Overall Conclusion (record in your notebook) • State outcome, yield for each step, overall yield for the two steps, proof of success and purity, possible sources of error, and any other information deemed necessary and relevant (i.e. spectral data as support). Also note how well recrystallization went (should compare TLC of crude vs. purified product). Experiment 2: Nitration: Synthesis of p-Nitroaniline The IR and NMR data for your aniline starting material are given below for your reference. IR of aniline (thin film) 1H NMR of aniline (90 MHz in CDCl3) s, 2H m, 3H m, 2H