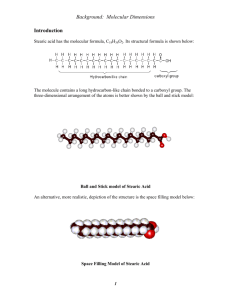
Stearic Acid Exemplar Lab
advertisement
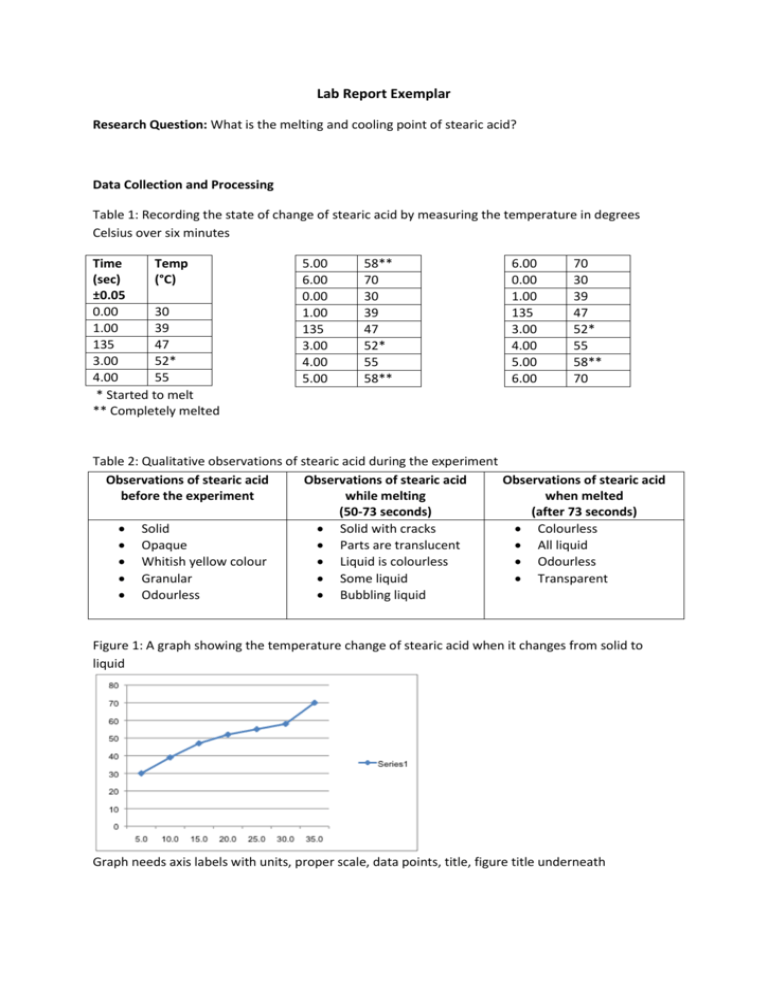
Lab Report Exemplar Research Question: What is the melting and cooling point of stearic acid? Data Collection and Processing Table 1: Recording the state of change of stearic acid by measuring the temperature in degrees Celsius over six minutes Time Temp (sec) (°C) ±0.05 0.00 30 1.00 39 135 47 3.00 52* 4.00 55 * Started to melt ** Completely melted 5.00 6.00 0.00 1.00 135 3.00 4.00 5.00 58** 70 30 39 47 52* 55 58** 6.00 0.00 1.00 135 3.00 4.00 5.00 6.00 70 30 39 47 52* 55 58** 70 Table 2: Qualitative observations of stearic acid during the experiment Observations of stearic acid Observations of stearic acid Observations of stearic acid before the experiment while melting when melted (50-73 seconds) (after 73 seconds) Solid Solid with cracks Colourless Opaque Parts are translucent All liquid Whitish yellow colour Liquid is colourless Odourless Granular Some liquid Transparent Odourless Bubbling liquid Figure 1: A graph showing the temperature change of stearic acid when it changes from solid to liquid Graph needs axis labels with units, proper scale, data points, title, figure title underneath Conclusion: In this investigation, the melting point of stearic acid was determined by heating solid stearic acid in a water bath using a Bunsen burner. Particles in solids have a repeating arrangement with strong intermolecular bonds holding them together (Ryan 11). Because of these bonds, the particles do not have freedom to move freely but can vibrate (Munn, 13 Sep. 2011). The process of melting is an endothermic reaction in which energy is added to a reaction. The Bunsen burner flame was the energy source in this experiment. Increasing energy increases the movement of particles, which will break bonds between solid particles (Ryan 11). As this occurs, the intermolecular bonds break, separating solid particles and changing their state to liquid, which have greater motion (Munn, 13.Sep. 2011). Temperature should remain constant from the beginning of melting until all solid stearic acid has changed phase to a liquid (Hyperphysics). In this experiment, stearic acid started melting at 52°C and was completely melted by 70°C (Table 1). This range in melting point is illustrated on Graph 1. According to research, the actual melting point of stearic acid is 69°C (Inchem). There is no plateau region shown on graph 1 because the temperature did not remain completely constant during the entire change of state. Temperature was not controlled perfectly, and reasons for this will be discussed in the evaluation. Evaluation: Sources of Error It was difficult to read the thermometer due to the condensation present on the test tube from the Bunsen burner heat. There was no specific mass of stearic acid measured for this experiment. Only one trial was done for this experiment. Could not control the amount of heat entering the reaction using a Bunsen burner. Temperature recording was not taken on the minute each minute. Solutions/Modifications Use an electronic thermometer that records temperature digitally, or logs data on the computer. Measure 5g of stearic acid using an electronic balance to make it possible for comparisons between group/class data. Repeat experiment 2 more times to obtain results for 3 trials in order to properly analyze data. Use a hot plate to maintain a set temperature for the heat. Have one partner count down the 5 seconds before the temperature reading is to be taken so other partner is prepared. Use data logging equipment that records and graphs data on the computer. Note: I wasn’t paying attention, I did not wear proper safety goggles, I didn’t stop the stopwatch at the correct time are not valid sources of error since YOU have control over those things. Bibliography: (MLA format – alphabetical order, single space between lines, double space between sources, all lines after the first are indented) Munn, Sharon. “Chemistry Review.” Science 10. Dubai American Academy. Dubai. 13 Sep. 2011. Powerpoint. “Phase Changes.” Hyperphysics. n.d. Web. 4 Oct. 2011. ˂ http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/phase.html ˃. Ryan, Lawrie. Chemistry for You. Cheltenham: Nelson Thornes, 2001. Print. “Stearic Acid Melting Point” Inchem. n.d. Web. 4 Oct. 2011. ˂http://www.inchem.org/documents/icsc/icsc/eics0568.htm˃.