Materials
advertisement

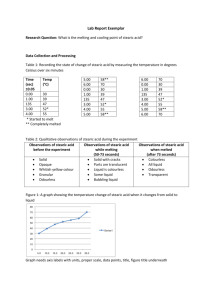
Name: Date: 10/15/13 Period: Explore: Stearic Acid Lab Objectives: Students will determine how temperature plateaus as a substance freezes Students will incorporate understanding of the kinetic theory of matter to identify and explain the behavior of solids, liquids, gases Be able to explain how a solid forms and how it melts (particles/energy) Purpose/Problem: How does a substance behave as it freezes? Hypothesis : ___________________________________________________________________ ___________________________________________________________________ Materials/ Methods- List all materials used in lab/ activity. Student Materials Safety goggles Test tube rack Thermometer Test tube with molten stearic acid Lab report worksheets/ data sheet Pen pencil Instructor Materials *Safety goggles *Hot plate *Glass beakers Procedures- Step by step instructions – Label each step 1, 2, 3, etc… 1. Get into pre assigned groups/ pairs, sit at lab station (desks) 2. Take out lab report worksheets/ data sheet and writing utensil 3. Collectively *Determine activity/ lab purpose *Address and discuss research *State hypothesis 1. Put on Safety goggles 2. Teacher will distribute molten stearic acid test tubes and place them in test tube rack 3. Record starting temperature, appearance and state of matter 4. Record and repeat step 6 every 30 seconds for between 10 and 15 minutes 5. Clean up lab stations(desks) *Return thermometers, test tubes, and goggles to instructor station *Wipe/ clean any dirty lab area 9. Work on/ complete Lab report questions * Create graph for temperature data 4. Experiment: Observations Freezing Liquid Lab/ Activity Data Sheet (10-15minutes) Elapsed time (sec) Start 0:00 0:30 1:00 1:30 2:00 2:30 3:00 3:30 4:00 4:30 5:00 5:30 6:00 6:30 7:00 7:30 8:00 8:30 9:00 9:30 10:00 10:30 11:00 Temperature *C Appearance /Observation Physical State 11:30 12:00 12:30 13:00 13:30 14:00 14:30 15:00 Analysis: Create a line graph of your data Discussion: Answer the questions to help you describe what happened in your experiment 1. Describe what happened to the stearic acid as it cooled down (ie: how did you know it was starting to freeze? _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 2. Was your hypothesis supported? Why or why not? _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 3. What state of matter is stearic acid at room temperature? _______________ 4. What do you think the freezing point of stearic acid is? _________________ 5. Describe what you think is happening to the molecules of stearic acid as it was freezing? _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 6. In the test tubes below to draw the particle arrangement of the stearic acid. LIQUID/MOLTEN SOLID 7. Based on your observations what type of solid did the stearic acid become, a crystalline solid or non- crystalline solid? Explain your answer _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 8. How does the stearic acid behave differently than water when it freezes? _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 9. In one sentence describe what you learned in this experiment. _______________________________________________________________ _______________________________________________________________