Melting and Freezing Lab
advertisement

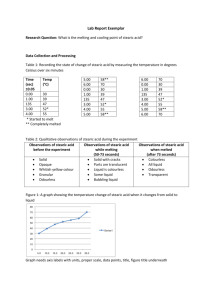
Melting and Freezing Lab Name:__________________ All matter is made of particles which are in motion. These particles arrange themselves in order to minimize their kinetic energy at the temperature and pressure of the environment. The phase of the matter at a specific temperature and pressure is a physical property of that matter. In this lab, you will observe melting and freezing. You will record what you think the melting point of a substance is and then determine the freezing point of the same substance. In the graph of freezing, a plateau of significant length will indicate the freezing point of the specific substance. Procedure: 1. Make a water bath heating apparatus with a 250-mL beaker with enough water to heat a test tube containing about 2 grams of stearic acid (make sure the stearic acid is below the water line). Suspend the test tube with a test tube clamp in the middle of the water so it is not touching the beaker. We will assume the temperature of the water bath to be the same as the temperature of the stearic acid. 2. Begin heating and record the temperature of the water every 30 seconds while recording all observations at each interval. (note: hold the thermometer so it is suspended in the middle of the water and not measuring the temperature of the hot glass) 3. When all of the stearic acid has melted, stop heating and begin cooling by adding ice to the water bath and recording the temperature every 10 second until the stearic acid completely solidifies. Remove water from the beaker if it starts to overflow. 4. Perform a second trial with the same sample of stearic acid and make necessary corrections. 5. Return the test tube with stearic acid to the test tube rack at the front of the classroom. Data / Analysis: 1. Make a quantitative and qualitative table for the melting portion of the lab with time, Kelvin temperature, and observations for both trials (don’t forget units and sig digs). 2. Make a graph for the freezing portion of the lab with time on the x-axis (horizontal) and Kelvin temperature on the y-axis (vertical) for both trials (don’t forget units and sig digs). Conclusions: 1. Give a kinetic definition of freezing in complete sentences. Is it an endothermic or exothermic (circle one) process. Is it an example of an increase or decrease (circle one) in entropy? 2. Give a kinetic definition of melting in complete sentences. Is it an endothermic or exothermic (circle one) process. Is it an example of an increase or decrease (circle one) in entropy? 3. According to your table of observations, what is the melting point of stearic acid for each trial in K, oC, and oF? (round answers to same number of decimal places as your measurement) 4. The actual melting point of stearic acid is 70.0oC; what is your percent error for each trial with 3 sig digs? 5. Using the actual melting point of stearic acid, how many Joules of heat is required to melt 56.13 g of stearic acid at 26.2oC if the specific heat of stearic acid is 3.28 J/goC? 6. How many calories of heat is your answer from #5? 7. Factor Label Method Problem – What is the mass in kilograms of a sample of stearic acid if it takes 16000 J of heat to melt the sample and energy required to melt one gram of stearic acid at its melting point is 34.8 cal/g? For your lab report – turn in this sheet along with melting tables and freezing graphs!