Stearic Acid
advertisement

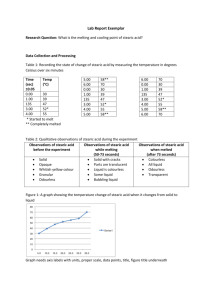
Lesson Plan: Stearic Acid Created by: In this lesson, students investigate how stearic acid undergoes a 2014 AACT Middle School phase change from solid to liquid and back from liquid to solid. Content Writing Team Temperature readings will be collected at one-minute intervals once the acid melts, the heat escapes, and the acid cools. Students are introduced to the idea that energy loss does not always result in a continuous temperature drop. Resource Type Lesson plan Grade Level Middle school Objectives By the end of this lesson, students should be able to Describe the reason that temperatures do not fall uniformly when a substance cools from liquid to solid. Determine the melting and freezing point of a substance through data collection and observation. Chemistry Topics This lesson supports students’ understanding of the following topics in chemistry: States of matter Freezing point Melting point Time Teacher Preparation: 30 mins Lesson: 80 mins Materials For each group: Test tube (16 x 150 mm) Stearic acid Hot plate Test tube rack Ring stand with clamp 1,000-L beaker Water Thermometer Test tube tongs Timer Safety Students will need to wear aprons and safety goggles. Do not directly touch stearic acid with your skin. If any acid gets on students’ skin, they should immediately alert you and thoroughly flush their skin with water. Exercise caution when using a heat source. Hot plates should be turned off and unplugged as soon as they are no longer needed. The test tube should be removed from the hot water bath with gloves or test tube tongs. Students should wash their hands thoroughly before leaving the lab. When students complete the lab, instruct them how to clean up their materials. Vocabulary Terms Melting Melting point Solidifying Freezing point Keywords Stearic acid, graph, temperature, melting point, freezing point, solidifying Teacher Notes MSDS for stearic acid: http://www.sciencelab.com/msds.php?msdsId=9927609 If the class is shorter than 80 mins, the lesson can be broken into two parts. Students will set up the experiment and melt the acid on day one. For day two, the teacher can melt the acid prior to class so the students can start right away with tracking the minute by minute temperatures as the acid cools and solidifies. Lower level: prep the graph and the materials so that everything is set up and the students just proceed with the experiment and data collection Higher level: Students can set up the entire lab and write their own step-by-step procedures. They can also decide what type of graph is best to show the temperature change of the acid cooling. Lesson Student Activity Sheet: Stearic Acid Engage - - Begin the class by having a short discussion about acids and their properties. Ask students what they know about acids and what acids can do and are used for. Move the conversation in this direction: did anyone shower/bathe in the last 24 hours? Tell the students that they may have used an acid while bathing. Describe stearic acid and what it is used for. [It’s a vegetable-derived waxy substance that is used as a hardening agent in soaps. It’s also used in candles.] Finally, ask students if all acids are liquids at room temperature. Explain that not all acids are liquids at room temperature and stearic acid is one of those examples. Hand out the Student Activity Sheet and explain what students need to complete. The graph and summary questions can be assigned as homework, depending on time constraints. Explore 1. Place 3 mL of stearic acid into a test tube using a scoop (the teacher may want to prepare test tubes ahead of time to save time or to prevent waste). 2. Fill 1,000 mL of water into the beaker and place onto the hot plate. Depending on the hot plate, this may need to be done sooner as some hot plates take a while to heat. 3. Suspend a thermometer in the center of the water in the beaker using the ring stand. Do not just place the thermometer into the bottom of the beaker as the heat from the hot plate will distort temperature readings. 4. Once the water reaches 50 oC, remove the thermometer from the water and lower the bottom half of the test tube into the center of the water. Do not allow any water to get into the test tube. 5. Completely melt the stearic acid and remove it from the hot water. The acid will be clear once the solid melts. Using the test tube tongs, remove the test tube and place into the test tube rack. Immediately place the thermometer into the acid and position it so that it can be read without being touched. It is important to not touch the thermometer or test tube while it cools to allow for proper data collection. 6. Once the temperature stops rising on the thermometer, begin recording and timing the temperature readings. 7. Continue to take the temperature of the acid every minute for 30 minutes 8. Once the acid becomes solid, do not remove the thermometer! It may break. Melt the acid again, removing the thermometer once it melts. Place the acid in the test tube rack to cool and rinse the thermometer off in the sink with hot water. If any stearic acid remains on the thermometer, the hot water should remove it from the thermometer. Explain 1. Fill in the temperature data table provided on the activity sheet. 2. Create a graph of temperature versus time. o On the graph: label the area where the acid is liquid, solid, and solidifying. [The flat area on the line graph is generally where the acid is a mixture of solid/liquid.] o Determine the freezing/melting point using the graph. [Again the flat area on the graph is where the melting/freezing point will be found.] 3. Once students have completed the activity, discuss the following as a class: 1. Why did the temperature remain constant for several minutes well above room temperature? [Energy loss goes to converting the liquid to solid, not to changing temperature.] 2. What happens structurally to the molecules of stearic acid as the substance cools? [The molecules slow down and begin to “stick” together, so they don’t have enough energy to bounce around in the liquid motion any more. They get closer together, which results in the solid state.] 3. What happens to the temperature of a bucket of ice as it melts into liquid water? [The ice should be at the freezing point of water because it’s a mixture of water and ice. As the ice melts, the entire system should remain at the freezing point of water until all of the ice melts. If the bucket is large enough, this could take hours. Despite the room temperature around the bucket and ice being much higher, the temperature of the melted water can’t increase until the entire process of melting is complete. This is similar to the stearic acid solidifying and the temperature remaining constant for several minutes even though the test tube and acid are surrounded by room temperature.] Elaborate 1. Fill a bucket with ice and add some room temperature tap water. 2. Insert a thermometer into the ice bath and record the temperature every minute until the ice melts. 3. Create a graph of temperature versus time and label the mixture of water and ice phase and the total liquid state. 4. What happens to the temperature of the water as the ice melts? [it hovers around freezing] 5. Why does the water maintain a temperature at/around freezing for so long despite the ice melting and the relatively warmer air temperature around the bucket? [The process of melting needs to be complete before the temperature can rise.] 6. How does this translate to a situation where a sealed thermos keeps ice cubes frozen in water for hours after it is removed from the freezer? [The sealed thermos traps the air within and the cold water and ice lower the air temperature. If the air temperature and the water remain at or near freezing, ice will remain as ice in the water for a longer period of time.] 7. How does this temperature versus time graph compare to your stearic acid graph? [This graph should begin with lower values before rising and plateauing. Finally the temperature will rise again after melting is complete. Depending on the amount of ice, the graph would indicate a much larger time period and an inverted line graph from the stearic acid. The melting point is also lower than steric acid’s melting point.] 8. It is possible to add the following to the above experiment: a. Place the melted stearic acid into a small beaker of water and track the temperature of the water simultaneously with the cooling acid. Note the results and when the water temperature stops increasing. [The water temperature should continue to increase for some time even as the stearic acid cools.] Evaluate Multiple Choice Items 1. Why does stearic acid remain a solid at room temperature? a. It is a weak acid b. The freezing point is higher than room temperature* c. The liquid evaporated out of the acid, leaving solid particles d. The acid is too dense to be a liquid 2. Why should the thermometer remain motionless in the acid as it cools? a. The liquid in the thermometer could get shaken and therefore the reading could change b. Moving the thermometer in the solidifying acid could cause the thermometer to break* c. Friction could be created with movement that could increase the temperature artificially 3. Why does the temperature remain constant during the state change from liquid to solid? a. The energy released from molecules packed together keeps the temperature constant* b. The thermometer does not show accurate readings as the substance changes states c. Energy release pauses as the substance changes states Open-Ended Questions 1. Why do you think the temperature of the melted stearic acid remained steady or near steady at a temperature well above room temperature for at least several minutes? [the energy loss involved molecules slowing and sticking together before the temperature dropped again. Also the bonding between the molecules sticking releases some energy into the system] 2. What was going on with the particles of stearic acid (on a very small level) that can help explain why the temperature didn’t drop consistently? [as energy escaped from the liquid, the particles came closer together to change from a liquid to a solid. During this transition, no temperature change is observed.] 3. Why did the temperature drop consistently as soon as it was removed from the warm water bath and then again at the end of the time period? [When all of a substance is in the same state, the temperature can change consistently to equalize to the temperature of its surroundings. But when a substance changes state, the temperature remains constant no matter what the surrounding temperature is.] Cross-Disciplinary Extensions Connect to Math Graphing; conversion between Celsius and Fahrenheit Connect to Reading/Writing Connect stearic acid to your home by researching what materials in your home contain stearic acid and how the stearic acid is necessary in the product, including what it does and what the product would be without it. Next Generation Science Standards This lesson supports the following: Practices of Science and Engineering Asking questions and defining problems Planning and carrying out investigations Analyzing and interpreting data Constructing explanations and designing solutions Engaging in argument from evidence Using mathematical and computational thinking Obtaining, evaluating, and communicating information Cross-Cutting Concepts Patterns Cause and Effect: Mechanism and Explanation Scale, Proportion, and Quantity Energy and Matter: Flows, Cycles, and Conservation Disciplinary Core Ideas, Grades 6-8 Physical science Solids may be formed from molecules, or they may be extended structures with repeating subunits (e.g., crystals). (MS-PS1-1) In a liquid, the molecules are constantly in contact with others. In a solid, atoms are closely spaced and may vibrate in position but do not change relative locations. (MS-PS14) The changes of state that occur with variations in temperature or pressure can be described and predicted using these models of matter. (MS-PS1-4) The term “heat” as used in everyday language refers both to thermal energy (the motion of atoms or molecules within a substance) and the transfer of that thermal energy from one object to another. In science, heat is used only for this second meaning; it refers to the energy transferred due to the temperature difference between two objects. (secondary to MS-PS1-4) The temperature of a system is proportional to the average internal kinetic energy and potential energy per atom or molecule (whichever is the appropriate building block for the system’s material). The details of that relationship depend on the type of atom or molecule and the interactions among the atoms in the material. Temperature is not a direct measure of a system's total thermal energy. The total thermal energy (sometimes called the total internal energy) of a system depends jointly on the temperature, the total number of atoms in the system, and the state of the material. (secondary to MS-PS1-4) Temperature is a measure of the average kinetic energy of particles of matter. The relationship between the temperature and the total energy of a system depends on the types, states, and amounts of matter present. (MS-PS3-3),(MS-PS3-4) The amount of energy transfer needed to change the temperature of a matter sample by a given amount depends on the nature of the matter, the size of the sample, and the environment. (MS-PS3-4) Energy is spontaneously transferred out of hotter regions or objects and into colder ones. (MS-PS3-3)