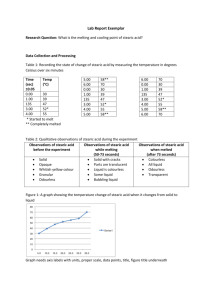
Background: Molecular Dimensions Introduction
advertisement
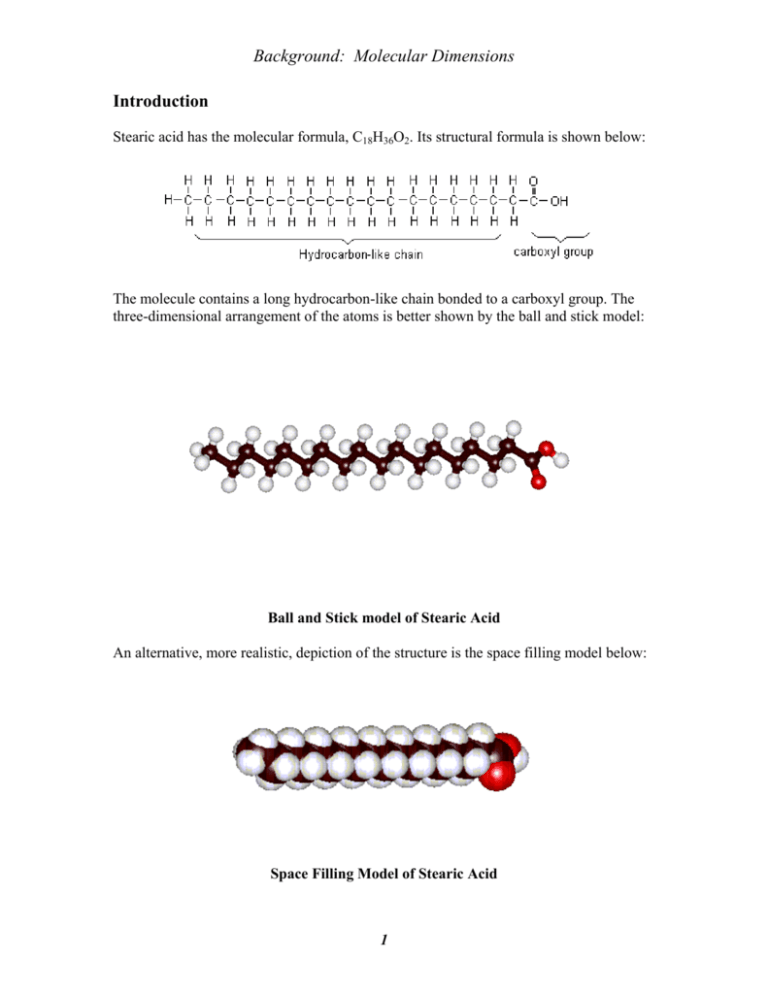
Background: Molecular Dimensions Introduction Stearic acid has the molecular formula, C18H36O2. Its structural formula is shown below: The molecule contains a long hydrocarbon-like chain bonded to a carboxyl group. The three-dimensional arrangement of the atoms is better shown by the ball and stick model: Ball and Stick model of Stearic Acid An alternative, more realistic, depiction of the structure is the space filling model below: Space Filling Model of Stearic Acid 1 Background: Molecular Dimensions The hydrocarbon-like chain is nonpolar, and so has little affinity for polar water molecules, and a low solubility in water. The carboxyl group is polar, is attracted to water molecules and has a strong tendency to dissolve in water. A shorthand representation of stearic acid is provided below. nonpolar "tail" O polar "head" The Stearic Acid Molecule - Shorthand Representation When stearic acid is added to water its molecules tend to accumulate on the surface of the water. The carboxyl group “head” dissolves in water and the hydrocarbon end “tail” sticks out away from the water surface. The nonpolar ends are attracted to each other but avoid interaction with the water molecules. If the amount of the stearic acid added is just sufficient to cover the water's surface, the stearic acid forms a layer which is just one molecule thick. This is referred to as a monolayer. In the monolayer, the molecules are arranged as shown in the figure below. Notice that the thickness of the monolayer is equal to the length of the stearic acid molecule. A magnified view of a stearic acid monolayer 2 Background: Molecular Dimensions Experimental Method In this experiment, a solution of stearic acid in hexane with a known concentration is added to water to just cover the surface. Once the solution is added, the hexane solvent evaporates leaving a film of pure stearic acid on the water surface as a monolayer. If V is the volume, in mL (or cm3), of the stearic acid-hexane solution needed to form a monolayer, and c is its concentration in g/cm3, then the mass of stearic acid in grams, m, in the monolayer is: m = cV If we assume that the density of the stearic acid in the film is the same as that of bulk stearic acid, dsa = 0.85 g/mL( or g/cm3), then the volume of the film, Vfilm, in cm3, is Vfilm = m/ dsa The volume of the film, in cm3, is also given by the product of the film thickness, t, in cm (the molecular length), and its area, A, in cm2: Vfilm = tA Equating the two expressions for the film volume and solving for t gives: molecular length = t = m/(A dsa) The cross sectional area of a stearic acid molecule is obtained from the total area of the film and the number of molecules in the film from the expression; number of molecules in the film = N = mNA / (MMsa) where NA is Avogadro's number( 6.022x1023atoms/mole) and MMsa is the molar mass of stearic acid (grams/mole).. The cross sectional area of a molecule is equal to the total film area, A, in cm2, divided by the number of molecules in the film: molecular cross sectional area in cm2 = A/ N 3 Background: Molecular Dimensions A Theoretical Calculation of Molecular Length Assuming that the length of a stearic acid molecule is approximately the same as that of a hydrocarbon molecule containing 18 carbon atoms, an estimate of the length of a stearic acid molecule can be calculated and compared with your experimental value. Be sure you show all your calculations in your Results section of your lab report and pay attention to significant figures. The following figure will be useful in making your estimation. In a hydrocarbon molecule in which all of the carbon - carbon bonds are single bonds, the bond angle is 109.5o and the average C - C bond length is 154 pm. The fully extended molecule has its carbon atoms in a zig-zag arrangement with the geometry shown below. Using your trigonometry skills, devise a way to calculate the length of the 18-atom molecule (shown below). Last modified on 7-15-09 by S. Phillips. 4