Stearic Acid Melting & Freezing Lab Report
advertisement

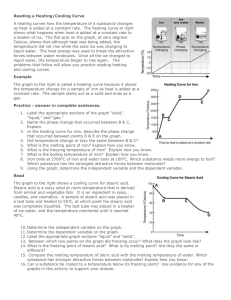
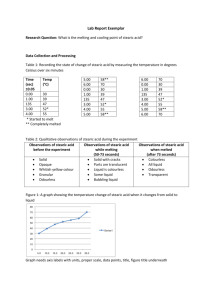
Melting and Freezing of Stearic Acid Lab Aaron Hile Partner: Isaac Terwilliger, Nick Powell Date: 2-28-11 Purpose: We will be observing melting and freezing points of an acid. Hypothesis: If we follow the procedure, then we will be able to find exactly the melting/freezing point of our acid. Materials: Ring Stand and Clamp, Test Tube Clamp, Bunsen Burner, Wire Gauze, Boiling Tube, Thermometer, Stop Watch, 250 mL beaker, stearic acid. Procedure: 1. We placed 150 mL of water in the 250 mL beaker. 2. We heated the water on the wire gauze above a medium flamed Bunsen burner just to the point where the water started to boil. 3. We kept the water the boiling but not vigorously. 4. We used a table to record the temperature of the stearic acid every minute until it reached 70 degrees Celsius. We made sure to note when the solid began to melt. 5. Once our solid was completely melted we used the ring stand to lift the tube from the hot water. We made sure to turn off our Bunsen Burner. 6. We recorded the temperature of the stearic acid every minute as it cooled until it reached about 30 degrees Celsius. We made sure to note the point where our acid begins to solidify. 7. We placed the boiling tubes with the thermometer still in them in the area instructed. We did not touch the thermometer. Solid Stearic Acid Melting to 70 ̊ C Melted Stearic Acid Freezing Temperature (C ̊) 45 ̊ 52 ̊ 58 ̊ 59 ̊ 60 ̊ 70 ̊ Minutes 1 2 3 4 5 6 Minutes 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Acid In Melting Process 80 70 50 40 30 Series 1 20 10 0 1 2 3 4 5 6 Minutes Acid In Freezing Process 80 Temperature Temperature 60 Temp (C ̊) 70 ̊ 65 ̊ 59 ̊ 56 ̊ 55 ̊ 54 ̊ 54 ̊ 54 ̊ 53 ̊ 53 ̊ 53 ̊ 53 ̊ 52 ̊ 52 ̊ 52 ̊ 52 ̊ 51 ̊ 51 ̊ 46 ̊ 40 ̊ 34 ̊ 30 ̊ 60 40 Series 1 20 0 0 2 4 6 8 10 12 14 16 18 20 Time Observations: When heated we saw the stearic acid warm up and melt into a liquid form, once it reached 70 ̊ C we let it cool (Freeze) into a waxy solid. Safety: We wore aprons and goggles to protect ourselves. We made sure that we turned the heating plate off when not in use. Conclusion: My hypothesis was correct. We were able to find the melting and freezing point of the stearic acid through simple procedure.