Quiz #25 Types of Chemical Reactions II with Answers
advertisement

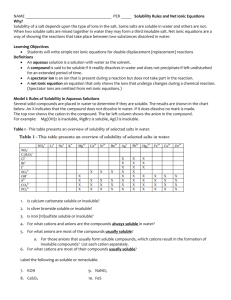
CP Chem.: Quiz #25 Types of Chemical Reactions II Metal Reactivity Series: lithium > rubidium > potassium > calcium > sodium > magnesium > aluminum > manganese > zinc > iron > tin > lead > copper > silver > platinum > gold Halogen Reactivity Series: F2 > Cl2 > Br2 > I2 Solubility Rules: 1. Nitrates and acetates are generally soluble. 2. Compounds of the alkali metals and the ammonium ion are generally soluble. 3. Chlorides, bromides, and iodides are generally soluble. EXCEPT the halides of Ag +, Hg22+, and Pb2+; also HgI2, BiOCl, and SbOCl. 4. Sulfates are generally soluble, EXCEPT PbSO4, Sr SO4, and BaSO4 are insoluble. CaSO4, Hg2SO4 are moderately soluble. The corresponding bisulfates are more soluble. 5. Carbonates, chromates, phosphates, and sulfites are generally insoluble. EXCEPT those of the alkali metals and ammonium ions are soluble. Many acid phosphates are soluble, i.e., Mg(H 2PO4)2 and Ca(H2PO4). 6. Sulfides are generally insoluble. EXCEPT those of the alkali metals and ammonium ion are soluble. The alkaline earth metals are soluble. Cr2S3 and Al2S3 decompose and precipitate as hydroxides. 7. Hydroxides are generally insoluble. EXCEPT those of the alkali metals and ammonium ion are soluble. The hydroxides of Ba, Sr, and Ca are moderately soluble. The hydroxide of Mg is only very lightly soluble. 8. Almost all ionic compounds containing NO2-, ClO4-, ClO3-, ClO2-, and ClO- are soluble. 9. All inorganic acids are soluble. Solubility of organic acids are variable. 1. What is the general form for a double-replacement reaction? (2 points) AX + BY ---> AY + BX AB ---> A + B A + BX ---> AX + B A + B ---> AB 2. What type of the chemical reaction describes the following chemical changes? Silver nitrate aqueous solution mixed with calcium bromide aqueous solution produced a white precipitate. (2 points) Single-replacement. Decomposition. Synthesis. Double-replacement. Complete combustion. 3. Which of the following ionic compound is soluble in water? (2 points) Hg2Br2 PbSO4 (NH4)2S Fe(OH)2 4. When aqueous solutions of aluminum nitrate and sodium carbonate are mixed together, ______. (2 points) a white precipitate of sodium nitrate is formed. nothing happens since all products are water soluble. a white precipitate forms first, then quickly disappears. a white precipitate of aluminum carbonate is formed. 5. Which of the following compound is soluble in water? (2 points) PbCO3 AgI BaSO4 Sn(NO3)2 6. In a chemical reaction, what is the relationship between the total mass of the reactants and the total mass of the products? (2 points) They must be equal. The mass of the products must be greater. There is no general relationship between them. The mass of the reactants must be greater. 7. Which of the following compound is insoluble in water? (2 points) Fe2S3 K2SO4 LiOH (NH4)3PO4 8. According to the law of conservation of mass, the total mass of the reacting substances is _____. (2 points) always more than the total mass of the products always less than the total mass of the products sometimes more and sometimes less than the total mass of the products always equal to the total mass of the products 9. Which of the following symbols means a substance is in water solution? (2 points) (w) (aq) (g) (l) (s) 10. After the correct formula for a reactant in an equation has been written, the ______. (2 points) formula should not be changed same formula must appear as the product subscripts are adjusted to balance the equation symbols in the formula must not appear on the product side of the equation CP Chem.: Quiz #25 Types of Chemical Reactions II 1. AX + BY ---> AY + BX 2. Double-replacement. 3. (NH4)2S 4. a white precipitate of aluminum carbonate is formed. 5. Sn(NO3)2 6. They must be equal. 7. Fe2S3 8. always equal to the total mass of the products 9. (aq) 10. formula should not be changed Version A Answer Sheet CP Chem.: Quiz #24 Types of Chemical Reactions I Metal Reactivity Series: lithium > rubidium > potassium > calcium > sodium > magnesium > aluminum > manganese > zinc > iron > tin > lead > copper > silver > platinum > gold Halogen Reactivity Series: F2 > Cl2 > Br2 > I2 1. complete combustion reaction 2. No reaction. 3. single-replacement reaction 4. No reaction. 5. synthesis reaction 6. Copper metal forms in aqueous solution of tin(II) nitrate. 7. incomplete combustion