Powerpoint 8.2 - Triton chemistry
advertisement

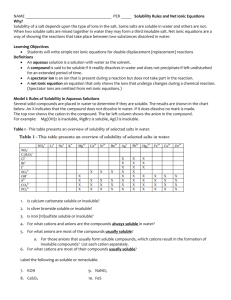
Solubility Cause sometimes stuff just won’t dissolve Solubility Dissolving is actually a very complicated process, if you want to look at the details. Start with a solvent (we’ll use water) and a covalent solute (call it sugar) Solubility After dissolving, it looks something like this: (the sugar molecules stay intact because the atoms are bonded together—each molecule just drifts away from its neighbors) Solubility With an ionic compound (say, NaCl), it’s actually a little bit more complicated. Solubility With an ionic compound (say, NaCl), it’s actually a little bit more complicated. Each ion has come apart from all the others, because only charge was holding them together—the water was able to slip in between. Three Steps Let's just look at the water molecules. Before dissolving, they look like this: Three Steps And after dissolving, they're like this—spread out. This is actually not favorable—water molecules want to be near each other. Step 2 The solute is even more dramatic. The ions get spread very far apart. This is very unfavorable; those charges want to be together. Step 3 Mix them together: this is favorable. Overall Dissolving consists of two unfavorable steps (pulling things apart), and one favorable one (putting them together). So which wins? Well....it depends. Some compounds dissolve, and some don't. There's no simple way to predict which will and which won't, so you just try them and see. You can come up with some guidelines, though. Solubility Rules For ionic compounds dissolving in water, there are two groups: Soluble: Nitrates Insoluble: Carbonates Alklali metals Hydroxides (NASH Sulfates Oxides CHOPS) Halides Phosphates Sulfides Solubility Rules For ionic compounds dissolving in water, there are two groups: Soluble: Nitrates Insoluble: Carbonates Alklali metals Hydroxides Sulfates Oxides Halides Phosphates Sulfides You just look at your compound and see if it has one of those groups: MgSO4 = soluble MgS = insoluble CaCO3 = insoluble Na3P = soluble CaCl2 = soluble Solubility Rules For ionic compounds dissolving in water, there are two groups: Soluble: Nitrates Insoluble: Carbonates Alklali metals Hydroxides Sulfates Oxides Halides Phosphates What about Na2CO3? It has an alkali (soluble), and a carbonate (insoluble)? Soluble Wins! Sulfides Exceptions As with anything in life, there are some exceptions. The notable ones are: Halides aren't soluble if they're paired with Pb2+ or Ag+ Sulfates aren't soluble if they're paired with Pb2+ or Ba2+ So AgCl, PbSO4, etc. will not dissolve. (in fact, insoluble silver halides are responsible for the entire fields of photography and film) Summary 1. Dissolving is actually a fairly complex process 2. Covalent solutes remain intact, but ionic ones separate 3. Some compounds dissolve in some solvents, some do not. 4. NASH CHOPS lets you make predictions about which will and won't. 5. These are not perfect rules, just guidelines based on experiments.