MS Bioswale Exploration Handout v1.1
advertisement

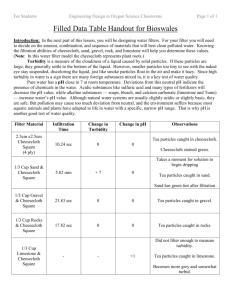
For Students Engineering Design in Oregon Science Classrooms Page 1 of 4 Exploration Handout for Bioswales Introduction: This activity helps you prepare for the next part of this lesson, when you will design water filters. You will need to decide on the amount, combination, and sequence of materials that will best clean polluted water. This exploration activity will help you explore the filtration abilities of individual materials. You can use what you learn in this activity when you design your water filter in the design activity. For each type of material (cheesecloth, soil, sand, pebbles, gravel, and limestone) you will determine how well it cleans pollutants from the water by measuring turbidity and pH before and after filtration. Turbidity is a measure of the cloudiness of a liquid caused by solid particles. If these particles are large, they generally settle to the bottom of the liquid. However, smaller particles too tiny to see with the naked eye stay suspended, discoloring the liquid, much like smoke particles float in the air and make it hazy. Since high turbidity in water is a sign there are many foreign substances mixed in, it is a key test of water quality. One way to measure turbidity is with Secchi depth which is what we will use. A low Secchi depth means a high turbidity, and a high Secchi depth means a low turbidity. Pure water has a pH close to 7 at room temperature, which is considered neutral. Deviations from this neutral pH indicate the presence of chemicals in the water. Acids — sulfuric acid and many types of fertilizers — decrease the pH value, while alkalines — soaps, bleach, and calcium carbonate (limestone and Tums) — increase water’s pH value. Although natural water systems are usually slightly acidic or slightly basic, they are generally safe. Pollution may cause too much deviation from the neutral pH, and the environment suffers because most aquatic animals and plants have adapted to life in water within a specific pH range. That is why pH is another good test of water quality. Article Notes: Write down four key facts about filtration, on a separate sheet of paper, from the Bioswale Article Handout. Background Paragraph: On the same sheet of paper, turn your article notes into a paragraph about filtration. Your paragraph should include a topic sentence, at least three supporting details, and a concluding sentence for five sentences total. Predictions: Answer the following questions. 1. Which material will have the slowest filtration rate? Why? 2. Which materials will lower turbidity the most and why? 3. Which material will raise pH the most and why? For Students Engineering Design in Oregon Science Classrooms Page 2 of 4 Procedure: 1. Get 600 ml of the dirty water solution. 2. Record the initial Secchi volume of the dirty water in Figure 1: Put the graduated cylinder on top. data Table #1, column #2. To measure turbidity, use a graduated cylinder and the picture of the Secchi disk at the end of this packet. Figure 2: Looking down into the cylinder, pour in the dirty water until you can no longer see the Secchi disk. 3. Measure then record the initial pH of the dirty water in data Table #2. Figure 3: Use a pipette as you get close to ensure your measurement is as accurate as possible. Record that level of water as the initial turbidity of the dirty water solution in Table #1. 4. You will be assigned one of the filter materials below. Prepare your assigned filter according to the instructions below: Cheesecloth: The cheesecloth represents plant roots in the soil. Take a square of cheesecloth and place it in the cap. Screw the cap onto the container, making sure that the cheesecloth does not bunch up. It should cover all the cap holes. Sand: Take a square of cheesecloth and place it in the cap. Spread a 1/3 of a cup of sand evenly in the container. Gravel: Take a square of cheesecloth and place it in the cap. Spread a 1/3 of a cup of gravel evenly in the container. Rocks: Take a square of cheesecloth and place it in the cap. Spread a 1/3 of a cup of rocks evenly in the container. Limestone: Take a square of cheesecloth and place it in the cap. Spread a 1/3 of a cup of limestone evenly in the container. 5. Place your plastic filter in a beaker. 6. Stir the contaminated water until its contents are evenly distributed, then pour 150 ml into your filter. Start the timer once all the water has been poured. When 100 ml of water has collected in the beaker below, stop the stopwatch and record the infiltration time in Table #3. 7. Let the remainder of the water filter. Record your observations of both the filter and the filtered liquid in Table #4. 8. Measure the Secchi volume of the filtered water and record in Table #1. 9. Measure the pH of the filtered water using a pH Strips, compare the color of the strip to the color key and record the pH in Table #2. 10. Dump your water solution in the sink and discard the dirty filter materials in the appropriate place. Wash out your plastic container, pipette, beaker and graduated cylinder. 11. Repeat steps 4 through 10 two more times for a total of three trials. 12. Find your average time, Secchi volume, and pH by adding up your three trials then dividing by three. Record the averages in the appropriate tables. 13. Subtract the initial Secchi volume from your average final turbidity to determine the average change in turbidity. Record the result in Table #1. For Students Engineering Design in Oregon Science Classrooms Page 3 of 4 14. Subtract the initial pH from your average final pH to determine the average change in pH. Record the result in Table #2. 15. Record your results on the class data sheet. 16. Clean up and return materials. Data Table #1: Turbidity Filter Material Initial Secchi Vol. (ml) Filter Material Initial pH Final Secchi Vol. (ml) Trial 2 Trial 3 Tria 1 Average Change in Secchi Vol. (ml) Average Change in pH (ml) Data Table #2: pH Trial 1 Trial 2 Final pH Trial 3 Data Table #3: Infiltration Time Filter Material Trial 1 Infiltration Time (seconds) Trial 2 Trial 3 Data Table #4: Observations Filter Material Filter Observations Water Observations Average Time For Students Engineering Design in Oregon Science Classrooms Page 4 of 4 Analysis Questions 1. Which of the five materials (cheesecloth, sand, gravel, rock and limestone) took the longest to filter the water? What might have caused this? 2. Which of the five filter materials lowered Secchi volume the most? What might have caused this? 3. For all the materials, your Secchi volume should have lowered (meaning the water solution became clearer) or remained the same. If it increased instead, what could be the source of experimental error? 4. What is the relationship between filtration time and Secchi volume? 5. Which of the five filter materials raised the pH? Why? 6. What is one trade-off associated with water filters? 7. What happens to the pollutants once they have been filtered? Are they still a danger to the environment? Why or why not? 8. Once water has been filtered like this, is it safe to drink? Why or why not? Figure 4: Secchi Disk