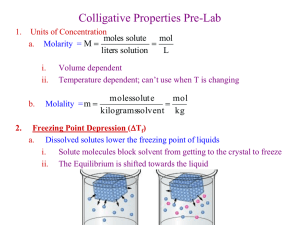
Activity 2: Frozen Solid
advertisement

Physical Science Institute Summer 2013 Frozen Solid MATERIALS (1) balance, top loading (7) test tubes, 20x150mm (1) container of fine ground kosher salt (2) temperature probes, stainless steel (1) 20-oz container of table sugar (1) Lab Quest2 data collection device (1) ring stand (1) buret clamp (2) plastic cups (20 oz) for ice and rock salt (1) Styrofoam cup, 20 oz (1) test tube rack (3) stoppers, rubber #2 (1) bottle, dispensing w/ flip-top 8oz, labeled “purified water” filled with purified water (1) bottle, dispensing w/ flip-top 8oz, labeled “ethyl alcohol” filled with ethyl alcohol PRE-LAB In this activity we will investigate the effect a solute has on the freezing point of water. Your group will be assigned one of the following solutes: ethyl alcohol (C2H6O) molecular mass = 46.0 table sugar (C12H22O11) molecular mass = 342 table salt (NaCl) molecular mass = 58.5 glycerin molecular mass = 92.0 1. a) Formulate a hypothesis about the how dissolving a solute affects the freezing point of water. Make it an “If…., then…” statement. b) What is your test variable in this experiment? What is your outcome variable? c) What controls are needed in this experiment? d) Give some examples of variables that are not important to control in this experiment. 2. One variable we need to control is the concentration of the solution. Since we have a method for counting molecules, we can control the number of molecules of solute per mass of water. It is found by a previous experiment that a solution of 9.2 g of glycerin in 100 grams of water has a freezing temperature -1.9 oC lower than freezing point of purified water. So that we can compare results between groups, we will keep the number of molecules per 100 grams of water constant for each solution. For the solute you’ve been assigned, calculate the mass you would need to get the same number of molecules as are contained in the 9.2 g of glycerin. PROCEDURE 1. Label a 250-mL beaker so your group can identify it. Make a solution in the beaker by dissolving the mass of your solute using the pre-lab calculations in #2 in 100 grams of purified water. Swirl the mixture until all the solute dissolves. 2. Turn the LabQuest2 on. In the LabQuest App, tap the Meter tab Mode: Time Based Interval: 1 s/sample Duration 900 seconds and set up the data collection parameters: 1 Physical Science Institute Summer 2013 3. Pour purified water into your test tube to a height of ~5 cm (2 inches). Stopper the test tube opening to prevent rock salt from getting into your sample. Lower the clamped test tube into the Styrofoam cup and layer ice and rock salt around the test tube. Remove the stopper and insert the temperature probe into the sample of distilled water. Start collecting data while stirring the sample. When your sample has gone through the freezing temperature, stop the data collection. Save your data on the LabQuest2. Go the “File” drop down and tap “Save…” Save your data as H2Otrial1 and an identifier (e.g., “H2Otrial1-RMexico”). Do another trial with the distilled water. You must have two trials whose freezing temperatures are within 0.5 oC of one another before you continue. 4. Rinse your test tube with a small amount of the solution you made in step 1). Repeat procedure 3) using your solution instead of distilled water. You should have two trials with the solution whose freezing temperatures are within 0.5 oC of one another. DATA f.p. trial 1 (oC) f.p. trial 2 (oC) Water, purified Solution CONCLUSIONS AND RESULTS 1. a. Average the freezing temperatures of the two trials for your group’s solution. b. Average the freezing temperature of the two trials of water. c. Calculate the change in freezing temperatures (t) for the two trials of your solution: i. t = average f.p. of solution – average f.p of water. 2. a. How did your findings compare to the hypothesis you formulated? b. How did your findings compare to other groups? 2