Freezing Point Depression and Molar Mass Determination
advertisement

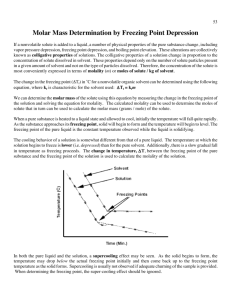
Academic Chemistry Lab: Freezing Point Depression and Molar Mass Determination Background: There are two parts to a solution. The substance being dissolved is the solute. The fluid that the solute is being dissolved in is the solvent. Colligative properties of solutions depend on the quantity of solute dissolved in a solvent rather than the identity of the solute. The freezing point of a solvent is lowered when a solute is added. This is called freezing point depression. The freezing point depression can be used to calculate the molar mass of the solute. For example, if sugar is added to water, the amount of water that can escape form the surface of the liquid decreases as compared to pure water. The larger sugar molecules interfere with the vaporization of the water molecules. The vapor pressure above such a solution would be lower than that of the pure water at the same temperature. Molecules of solutes such as sugar, block the surface of the solvent, thus preventing as many molecules from evaporating. The decrease in vapor pressure results in an increase in the boiling point and a decrease in the freezing point. In this study you will determine the depressed freezing point of naphthalene when an unknown solute is added. You will then determine the molar mass of the solute. The decrease in freezing point (∆Tf) when a solute is dissolved is proportional to the concentration of solute. The solvent has a freezing point depression constant Kf. This represents the number of degrees the freezing point will change if 1.00 mole of solute is added to 1.00 kg of solvent. Kf is different for different solvents. Kf for water is 1.86 ºC kg/mole, whereas Kf for benzene is 5.12 ºC kg/mole. The measurement of freezing point depression (∆Tf) is routinely used for the determination of the molar mass of unknown solutes. The following equation can be used to calculate molar mass. Molar Mass = (Kf)( gsolute) (∆Tf )(kgsolvent) For example: The freezing point depression constant for benzene is 5.12 ºC kg/mole. When 1.08g of unknown solute is dissolved in 10.02g of benzene (the solvent), the solution freezes 4.60 ºC lower than pure benzene. Calculate the molar mass of the unknown solute. (5.12 ºC kg/mol)(1.08g) Molar mass = ------------------------------- = 120g/mole (4.60ºC)(0.01002kg) Materials 13x100mm test tube Hot plate Lauric acid one hole split stopper Computer unknown solute thermometer probe Vernier software test tube clamp two 100mL beakers beaker tongs ring stand Name _____________________________________ Period _______ (10 points) Lab Partner: ________________________________ Academic Chemistry Lab: Freezing Point Depression and Molar Mass Determination Procedure: 1. Place two 100mL beakers about ¾ full of water on a hot plate and heat to boiling. 2. Clean and dry a 13x100mm test tube. Mass of this test tube is _________________________. 3. Fill the test tube w/ ~ 8grams lauric acid and record the mass. ________________________ 4. On a folded paper, mass approximately 1g of unknown solute to 0.001g. Also add this to the test tube and record the mass. ___________________________ 5. Clamp the test tube in the hot water bath and allow the contents to melt. 6. Plug the temperature probe into Channel 1 of the LabPro interface. Connect the LabPro to the computer using the proper cable. 7. Modify Logger Pro to collect data for 600 seconds (10 minutes). This can be done by selecting experiment/data collection. 8. When all the solution has melted (~ 60°C), use tongs to carefully remove the beaker and test tube from the hot plate, insert the temperature probe and stir. Using a split 1 one holed stopper, adjust the temperature probe so it does not touch the walls of the test tube. Begin collecting data, and allow the water and test tube to slowly cool. 9. The freezing point of this solution can be determined by finding the y-coordinate at the top of the small “blip.” This is also the point where the solution starts to cloud over. If you are satisfied with you data print a copy of this graph for each member of your lab team. If your data is poor, reinsert the test tube in the second boiling beaker to melt the sample again and cool a second time. 10. To clean-up: place the test tube in the second beaker of boiling water. Once the liquid has melted, remove the probe and place it directly in the beaker of boiling water. Wipe the probe off while it is still hot and return it to storage. Quickly pour the test tube of hot solution into the waste jug. Now completely fill this test tube with water and place it in the beaker of boiling water that is in the hood. DATA TABLE (1 point each block) smooth calculation for molar mass solute (2 pts): Mass Lauric acid Mass unknown solute Freezing Point of pure Lauric acid 44.0 ºC Freezing point of solution ∆Tf (change in temperature) Kf for Lauric acid 3.9 ºC kg mol Note: Temperature vs time graph is worth 2 points.