Freezing Point Lab
advertisement

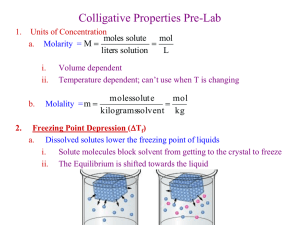
Determination of an unknown by freezing point depression Introduction Colligative properties are properties of a solution that depend on the collective effects of solute particles in the solution. These properties include the elevation of the boiling point of a solution compared to the pure solvent, the depression of the freezing point of a solution as compared to the pure solvent, reduction of the vapor pressure and the way osmotic pressure controls osmosis. The colligative properties of a solution depend on the quantity of the solute present in the solution and not on the chemical nature of the solute. Remember in a solution, which can be a solid, liquid, or a gas, the component that is in the greater quantity is the solvent and the other components of the solution are called the solutes. Colligative properties occur because of the intermolecular forces that ate operating between the solute and solvent in the solution. In this lab we will use the fact that a solution has a lower freezing point as compared to the pure solvent to determine the molecular weight of the solute in the solution. Using the colligative properties of solutions to experimentally determine the molar mass of the solute added to a solution the identity of the unknown solute can be determined. The decrease in the freezing point is directly proportional to the concentration of the solution expressed in molality. Or stated another way, the freezing point change between the pure solvent and the solvent in solution is proportional to the molality of solute in solution where molality is equal to the number of moles of solute particles per kilogram of solvent. ∆𝑇𝑓 = 𝑘𝑓 𝑚 Here, ΔTf stands for the change in freezing point between the freezing point between the freezing point of the solution (Tf) and the freezing point of the pure solvent, and kf is the molal freezing point depression constant, a proportionality constant, which is different for each solvent. In this experiment you will determine the freezing point of pure benzophenone and then measure the change in the freezing point when an unknown solute is added to the pure benzophenone. Using the following set of equations, the identity of the unknown can be determined by calculating its molecular weight and comparing its molecular weight to the compounds listed in Table 1. Substance Water t-butanol Cyclohexane Naphthalene Acetic acid Camphor Benzoic acid Benzophenone Benzene MW (g/mol) 18 74 84 128 60 152 122 182 78 F.P. (°C) 0.0 25.5 6.5 80.2 16.6 179 122.4 48.1 5.5 Kf (°C/m) 1.86 9.1 20.1 6.9 3.9 39.7 9.80 5.12 B.P. (°C) 100 Kb (°C/m) 0.52 80.7 217.7 118.3 204 249.2 2.79 5.80 3.07 5.61 80.1 2.53 ∆𝑇𝑓 = 𝑘𝑓 𝑚 𝑚= 𝑚𝑜𝑙𝑒𝑠𝑠𝑜𝑙𝑢𝑡𝑒 𝑘𝑔𝑠𝑜𝑙𝑣𝑒𝑛𝑡 𝑚𝑜𝑙𝑒𝑠𝑠𝑜𝑙𝑢𝑡𝑒 = 𝑔𝑠𝑜𝑙𝑢𝑡𝑒 𝑀𝑊 Start by solving for molality using the first equation, you will have measured ΔT. Then solve for the moles of the unknown using the second equation and finally solve for the molecular weight of the unknown using the third equation. Experimental Setup Place a beaker with 400 mL of water on a hotplate. Heat the water to between 60 – 70 °C. Temperature needs to be higher than 55 °C, but the exact temperature is not important, and you do not need a boiling water bath. Place an 8 inch test tube into the water bath. Make sure the test tube is not touching the bottom of the water bath; you can do this by clamping the test tube a ring stand. Get a two-hole stopper. Place a thermometer in one hole and the metal stirring rod in the other hole. Place the stopper in the test tube. Experimental Procedure Weigh out a 10g sample of benzophenone and record the actual mass. Add the benzophenone to the test tube. Wait for the sample to melt. Remove the test tube from the water bath. Watch closely for the first sign of crystallization. You will need to stir periodically as the temperature is decreasing. Record the temperature of the first visible crystallization. Place the test tube back in the water bath and repeat the procedure. This is the freezing point of the pure solvent. You must have at least two trials. Report the average freezing point. Weigh out a 1g sample of the unknown assigned to your group. Add this to the test tube containing the benzophenone. Allow the mixture to melt, stir the solution to insure that the entire unknown has dissolved in the benzophenone. Remove the solution from the water bath, continue stirring the solution, and watch for crystallization. Record the temperature of the first crystallization. This is the freezing point of the solution. Repeat. You should have two trials. Add a second 1g sample of the unknown to the solution in the test tube. Repeat the procedure of melting and recrystallization. Record the temperature of crystallization. Repeat. You should have two trials. Calculate the molecular weight of the unknown sample for trial 1 and trial 2. Identify the identity of the unknown based on the information found in Table 1. Clean Up To clean your test tube, first melt the solution in the hot water bath. Pour the liquid solution into the benzophenone waste container. Do not put pure benzophenone or the benzophenone solution down the drain. Wipe the stirring wire and thermometer with a paper towel that has been wetted with acetone. Put the used paper towel in bin in the hood. Rinse the test tube with a small amount (2 to 5 mL) of acetone. Pour this into the waste container. Repeat the acetone rinse.