Determining the Molar Mass of an Unknown
advertisement

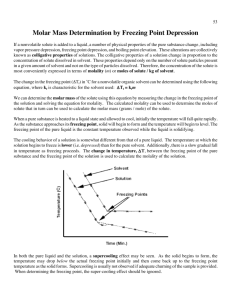
AP CHEMISTRY Determining the Molar Mass of an Unknown Substance by Freezing Point Depression In chemistry, the molar mass is used for a number of calculations. However, if we synthesize or discover a new compound, the molar mass needs to be determined experimentally. There are a number of methods to do so. The most modern and precise method is mass spectroscopy. The older methods, vapor density, osmotic pressure, freezing point depression, and boiling point elevation rely on colligative properties of solutions. Solutions are homogeneous mixtures of a solute and a solvent. Some properties of a solvent change after a solute has been added. The changes that solely depend on the number of particles that are added to the solvent are known as colligative properties. Vapor density, osmotic pressure, freezing point, and boiling point of a substance are colligative properties. When salt is added to water, its freezing point is lowered and this properties is used when salt is spread on mountain roads in the winter to avoid ice. On the same token, the salt presence increases the boiling point of water, so that in cooking some recipe will call for salt to be added before water boils so that the item in the water will cook at higher temperatures. For the freezing point, the changes in temperature are directly related to the amounts of solute according toe the following equation: T = iKfm i is the Van’t Hoff factor which represents the number of particles that a solute produces when it goes in solution. i value is equal to 1 when a solute such as glucose does not dissociate in multiple particles. i is equal to 2 when the solute dissociate into two particles upon dissolving such as NaCl. m is molality, one of the various ways to express concentrations. Molality is the number of moles of solute per kg of solvent (NOT SOLUION!) Kf is the cryoscopic constant specific to the solvent and it can be found listed in a handbook such as the Handbook of Chemistry and Physics. The larger the value of is, the easier is to see a change in the freezing point of a solution with that specific substance as solvent. The table below shows some commonly used solvents. Solvent Formula Normal Freezing Kf point (0C) (0C . kg/mol) Water H20 0.0 1.86 Ethylene glycol C2H6O2 -114.6 3.11 Acetic acid C2H4O2 16.6 3.63 Benzene C6H6 5.5 5.07 Cyclohexane C6H12 6.6 20.8 Lauric acid C12H24O2 43.2 3.9 Pre-lab questions Solve the following problems: 1. What would be the freezing point of a solution containing 5 g of sugar dissolve in 100 mL of acetic acid (density of acetic acid is 1.049 g/mL)? 2. What is the freezing point water after 5 g of NaCl have been added to it 3. A solution of 50 g of unknown substance dissolved in 200 g of ethanol is found to have a freezing point of -120 0C. Determine the molar mass of the unknown substance (assume i=1). In this lab, we will determine the molar mass of an unknown substance by determining the freezing point depression of lauric acid (C12H24O2) also known as n-dodecanoic acid. The freezing point of a substance is usually determined by constructing a heating/cooling curve for that substance. To that purpose the substance is allowed to slowly freeze (solidify) while recording the temperature at very frequent and regular intervals. Sometimes a phenomenon called “supercooling” can interfere with the determination of the freezing temperature. Supercooling happens when a liquid temperature is below its freezing point; it looks like a dip in the cooling curve of a substance (see graph below). http://wwwchem.csustan.edu/chem1112/ Another factor that can interfere with the accurate determination of the freezing temperature of a substance is the a relative broad spectrum of temperatures at which many substances solidify. To minimize these difficulties heating/cooling temperatures need to be taken when cooling very slowly and continuously stirring the substance. Materials Lauric acid Test tube Two-hole stopper Temperature probe (stainless steel by Vernier) Thermometer 400-mL beaker and 250 mL beaker Water Heat plate Thin glass stirrer Ring stand and tube clamp Procedure Freezing point of the pure solvent 1. Place a beaker with about 300 mL of water on a hot plate and start heating up the water at a setting of about 6. Bring the water temperature to about 70-75 0C. 2. Lower the heat (to about 3.5) so the water bath stays at this temperature. Make sure that the water level remains above 200 mL 3. Fill the other beaker with 200 mL water and leave it at room temperature 4. Set above it a ring stand with a tube holder above the beaker with water at room temperature ready to use later as shown in diagram 5. Mass the empty tube and record the mass 6. Using weighing paper, mass about 7 g of lauric acid. 7. Record the exact amount you took (it does not need to be exactly 7 g!) 8. Place the lauric acid sample in a tube. Seal it with the stopper and place it in a warm water bath (DO NOT go above 70-75 0C!). Let it melt completely 9. While melting prepare the temperature recording instrumentation a. Turn on the computer. WARNING! THE COMPUTERS ARE OLD AND RUN VERY SLOWLY, DO NOT USE ANYTHING ELSE BUT THE PROGRAM LoggerPro. b. Plug the 6-volt power supply for the LabPro the instrument in the outlet, shortly after plugging the power supply into the outlet, the interface will run a short self test and you will hear a few beeps. c. Connect USB cable to the LabPro instrument and to the computer d. Attach the temperature probe to the side of the instrument ion Channel 1. Lay the probe on the desk e. Double click on LoggerPro 3.8.4 f. When the program is running you should see that it detected that the temperature probe is there. To test that is works, just hold the probe in you hand and look at the temperature changing on the screen g. Program your experiment using the experiment drop down menu i. Click on change units, it should show LabProCh1 stainless steel and then temperature. Make sure it is in Celsius ii. Click on Mode; it will open up a dialog menu 1. Mode: time base 2. Length: 1500 sec (20 min) 3. Sampling rate use the sec/sample: 10/sample (which means record the temperature every 10 sec) iii. Whenyou are ready with your Lauric acid sample (see below), click Data collection to start your experiment 10. When completely melted (wait at least two minutes after is melted) place the stirrer and the temperature probe in the tube. 11. Move the tube to the cold water bath and start collecting data and let it run its course (you cannot stop it!). While collecting make sure to stir the lauric acid, however, when it solidifies do not attempt to remove the stirrer or the probe, the latter will be damaged! 12. Place tube back into hot water bath and let the lauric acid melt again 13. Save your data as follow: a. Under File drop down menu, click on Export as b. Choose InspireData(CVS)files c. Save it on the desktop in a newly created folder with your name or the name “FreezingPoint” d. Transfer your file to your memory stick/ flash drive Freezing point of the solution 1. Using weighing paper, mass about 0.7 g of the unknown substance. Record the exact amount of the unknown you use 2. Carefully transfer the unknown substance into the tube with the melded lauric acid from the previous part 3. Using the stirrer make sure that the unknown substance is fully mixed with the lauric acid. (NOTE: if the mixture is not fully mixed, the two substances will layer, and the unknown will NOT melt) 4. Reconnect the probe to the instrumentation and repeat experiment as in part 1. 5. Repeat with unknown substance another two times using about 1.4 g the 2nd time and 2.1 g the 3rd time Data Analysis 1. Transfer data to your flash drive 2. Use Excel to graph your data on one graph with 4 different color or symbol for each data set. 3. Determine the freezing temperature for each trial, label them on the graph 4. Calculate the T for each trial 5. Using the information give in the introduction calculate the molality of each solution 6. Calculate the experimental molar mass of the unknown substance. 7. Since in another experiment it was found that the empirical formula for the unknown compound is C7H6O2, determine the molecular formula for this compound. 8. Using the internet find the name for this compound 9. Calculate the percent error