alcohol-ether-2
advertisement

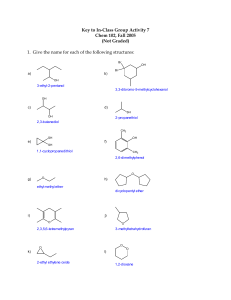
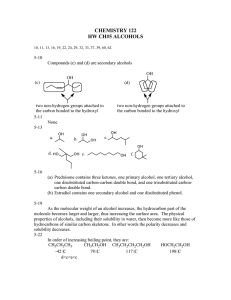
WORKSHEET-5 ALCOHOL-ETHER 8. Which of the following statements is incorrect ? MULTIPLE CHOICE QUESTIONS 1. The isomer of n-butyl alcohol is A) When oxidized, primary alcohols produce aldehyde. A) butanoic acid B) isopropyl alcohol C) ethyl methyl ether D) 2-butanol B) When oxidized, secondary alcohols produce ketone. E) none of them C) Alcohols produce hydrogen with sodium metal. 2. The oxidation of a secondary alcohol produces D) Alcohols form esters with acids. A) a ketone B) an organic acid C) an ether D) an aldehyde E) an ester E) Aldehydes and alcohols are isomers. 9. 3. Fermentation of glucose gives What is the product of the following reaction ? C6H5ONa + CH3I ........... A) carbon dioxide and water A) alcohol B) ether C) aldehyde D) ketone E) acid B) carbon monoxide and alcohol C) carbon dioxide and methanol 10. What is the organic substance which will be obtained through the oxidation of 3-methyl-2butanol ? D) carbon monoxide and ethanol E) carbon dioxide and ethanol A) ether B) aldehyde C) ketone D) acid E) ester 4. Which one(s) react(s) with sodium metal to produce hydrogen gas ? I. C2H5OH II. C2H6 III. CH3-CH(OH)-CH3 IV. CH3OCH3 11. Which one of the following compounds reacts with sodium but not with zinc ? A) CH3-O-CH3 B) CH3CHO C) CH3COOH D) CH3COCH3 E) CH3-CH2-CH2-OH 12. What is the name of the compound? A) I B) I-II C) I-III D) II-III E) II-IV 5. Which one(s) give(s) aldehyde when it is oxidized ? I. CH3OH II. C2H5CHO III. CH3-CH(OH)-CH3 IV. CH3COCH3 CH2-CH-CH-CH2-CH3 | | | OH CH3 OH A) 1,3-pentanediol B) 2-methyl-1,3-pentanediol C) sec-hexyl alcohol D) 2-methyl-1,3-hexanediol E) 1,3-hexanediol A) I B) I-II C) II D) II-IV E) III-IV 6. What is the product of the reaction between sodium methoxide and ethyl iodide? 13. Which one of the following compounds gives ethyl methyl ketone when oxidized ? A) 1-butanol 7. A) methane B) propane C) 2-propanol D) 1-propanol E) ethyl methyl ether B) 2-butanol C) 2-propanol D) t-butyl alcohol E) 2-methyl -1-propanol 14. Ethers and alcohols can be isomeric. How many alcohol and ether isomers does the molecular formula C3H8O have ? Given the reactions; H2SO4 CH3-CH2-CH2-OH Z + ......... 1800C Z + H2O Y What is the name of the product, Y ? A) propene B) propane C) 2-propanol D) 1-propanol E) methyl ethyl ether A) 1 B) 2 C) 3 D) 4 E) 5 WORKSHEET-5 ALCOHOL-ETHER SUPLEMENTARY QUESTIONS 1. Name the following compounds. a) C6H5CH2OH b) CH3CH2CH2CH2OH c) (CH3)2CHOCH(CH3)2 d) CH2=CHOCH=CH2 e) CH2CH2CH2OH | Br 2. 3. 4. f) CH2CH2CH2CH2 | | OH OH Write a structural formula for each of the following compounds. a) cyclohexanol b) n-pentyl alcohol c) 3-buten-2-ol d) 2-methyl-2-butanol e) 2-methoxy butane f) ethyl vinyl ether g) methoxy benzene Write all isomers of the compound having the formula, C4H10O. And name them. Also classify alcohol ones. Arrange the following compounds in order of increasing solubility in water. hexane, glycol, methanol, 1-pentanol 5. Arrange the following compounds in order of increasing boiling point. butane, 1-butanol, diethyl ether, glycerol