Average Atomic Mass Practice
advertisement

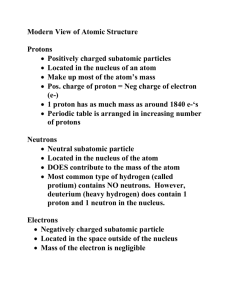
Name:________________________________ Period:________________ Date:______________ Average Atomic Mass Practice Average atomic mass is the weighted average of the masses of all the isotopes of that element. A weighted average reflects both the mass and the abundance of the isotopes as they occur in nature. To watch a YouTube video on this topic, search YouTube for: atomic mass introduction dewitt Formula Average atomic mass = (Atomic mass of isotope #1) x (Abundance of Isotope #1) + (Atomic mass of isotope #2) x (Abundance of Isotope #2)… and so on for each isotope Example: In nature, chlorine consists of two isotopes: Isotope Cl-35 Cl-37 Atomic mass 34.97 amu 36.97 amu Abundance (%) 75.8 % 24.2 % Abundance (as decimal) 0.758 0.242 To calculate the average atomic mass, multiply each atomic mass by the percent abundance (as decimal) and add them together. (34.97 amu) x (0.758) + (36.97 amu) x (0.242) = 26.51 + 8.95 = 35.46 amu Practice Problems Fill in the missing information and calculate the average atomic mass of each element (show your work). 1. Carbon Isotope Atomic Mass % Abundance C-12 12.00 amu 98.93% C-13 13.00 amu 1.07% Abundance as decimal Show your work : Average Atomic Mass of Carbon=__________ 2. Potassium Isotope K-39 Atomic Mass 38.96 amu % Abundance 93.26% K-40 39.96 amu 0.01% K-41 40.96 amu 6.73% Abundance as decimal Show your work : Average Atomic Mass of Potassium=___________ For the next two problems, construct the table yourself and solve the problem. 3. Three isotopes of argon occur in nature, Ar-36, Ar-38, Ar-40. Calculate the average atomic mass of argon given the following: Ar-36 (35.97 amu; abundance = 0.337 %), Ar-38 (37.96 amu; abundance 0.063 %), and Ar-40 (39.96 amu; abundance 99.6 %). 4. Rubidium has two common isotopes, Rb-85 and Rb-87. What is the average atomic mass of rubidium given the following: Rb-85 (84.911 amu, abundance = 72.2%) and Rb-87 (86.91 amu, abundance = 27.83%) 5. Without doing any math, are there more Bromine-79 atoms or more Bromine-80 atoms on earth? (Hint: look at the periodic table.) How do you know?