Average Atomic Mass Wksht
advertisement

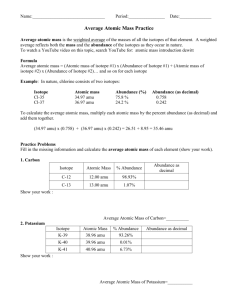
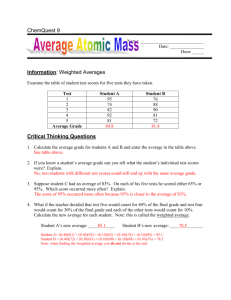
Name______________________ Date_______________ Chemistry Worksheet: Average Atomic Mass 1. Explain why atoms have different isotopes. In other words, how is it that helium can exist in three different forms? 2. Rubidium has two common isotopes, 85Rb and 87Rb. If the abundance of 85Rb is 72.2% and the abundance of 87Rb is 27.8%, what is the average atomic mass of rubidium? 3. Uranium has three common isotopes. If the abundance of 234U is 0.01%, the abundance of 235U is 0.71%, and the abundance of 238U is 99.28%, what is the average atomic mass of uranium? 4. Titanium has five common isotopes: 46Ti (8.0%), 47Ti (7.8%), 48Ti (73.4%), 49Ti (5.5%), 50Ti (5.3%). What is the average atomic mass of titanium? 5. Find the average atomic mass for Li if 7.5% of Li atoms are 6Li with a mass of 6.0151223 amu and 92.5% are 7Li with a mass of 7.0160041 amu. Name______________________ Date_______________ 6. Find the average atomic mass for B if 19.9% of B atoms are 10B with a mass of 10.0129371 amu and 80.1% are 11B with a mass of 11.0093055 amu. 7. Find the average atomic mass for Cl if 75.78% of Cl atoms are 35Cl with a mass of 34.96885271 amu and 24.22% are 37Cl with a mass of 36.96590260 amu. 8. Find the average atomic mass for Mg if 78.99% of Mg atoms are 24Mg with a mass of 23.9850419 amu, 10.00% are 25Mg with a mass of 24.9858370 amu, and 11.01% are 26Mg with a mass of 25.9825930 amu. 9. There are 2 isotopes of copper that occur naturally; 63Cu and 65Cu. The 63Cu atoms have a mass of 62.929601 amu and the 65Cu atoms have a mass of 64.927794 amu. What is the percent natural abundance for each isotope? 10. There are 2 isotopes of gallium that occur naturally; 69Ga and 71Ga. The 69Ga atoms have a mass of 68.925581 amu and the 71Ga atoms have a mass of 70.924707 amu. What is the percent natural abundance for each isotope?