29 Medical Gases - A Prescriber`s View and Future Trends
advertisement

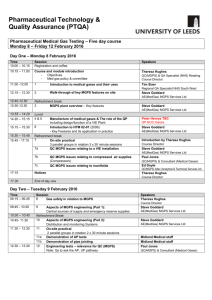
Pharmaceutical Technology & Quality Assurance (PTQA) Pharmaceutical Medical Gas Testing – Five day course Monday 9 – Friday 13 February 2015 Day One – Monday 9 February 2015 Time 10:00 – 10.15 Session Registration and coffee Speakers 10:15 – 11.00 Theresa Hughes Course Director 11.00 -12.00 Course and module introduction Objectives Med gas policy & committee 1 Introduction to medical gases and their uses 12.00 – 12.30 2 12:30-12.45 12.45-13.30 Refreshment break 3 MGPS plant overview – Key features 13.30 – 14.15 Lunch 4&5 14.15 – 15.00 15:00 - 15:15 15.15 – 15.45 15.45 - 17:15 Walk-through of key MGPS features on site Manufacture of medical gases & The role of the QP including design/function of a VIE Plant Refreshment break 6 Introduction to HTM 02-01 (2006) - Key Features and its application in practice 7 On-site practical 3 parallel groups in rotation 3 x 30 minute sessions 7a QC MGPS issues relating to a VIE installation 7b QC MGPS issues relating to compressed air supplies (Compressors) 7c QC MGPS issues relating to manifolds 17:15 Notices 17:30 End of day one Tim Sizer Regional QA NHS Bristol Andy Capper Authorised Engineer, MGPS Services Ltd Andy Capper MGPS Services Ltd Peter Henrys BOC Gases Andy Capper MGPS Services Ltd Introduction by Theresa Hughes Course Director Andy Capper MGPS Services Ltd Ian Buckingham QC (MGPS) Quality Control North East Ed Doyle Altec (Analytical & Technical) Services Ltd Theresa Hughes Course Director Day Two – Tuesday 10 February 2015 Time 09.00 – 09.45 Session 8 Gas safety in relation to MGPS 09:45 - 10:30 9 10:30 – 10:45 10:45- 11:30 11.30 – 12.30 Aspects of MGPS engineering (Part 1): Central sources of supply and emergency reserve supplies Refreshment Break 10 Aspects of MGPS engineering (Part 2): Distribution and monitoring Systems 11 On-site practical 2 parallel groups in rotation 2 x 30 minute sessions 11a Demonstration of AP tests 11b Demonstration of pipe jointing Speakers Theresa Hughes Course Director Andy Capper, Midland Medical staff Andy Capper, Midland Medical staff Midland Medical staff Midland Medical staff Day Two – Tuesday 10 February 2015 continued…. 12.30 – 13.15 12 13.15 – 14.00 Lunch 14.00 – 14.30 13 14:30 - 16:30 Engineering tests – relevance for QC (MGPS) Note: Qs to ask the AP, UP pathway Introduction to role of QC MGPS In relation to HTM 0201 14 Workshop sessions 3 parallel groups in rotation 3 x 40 minute sessions Refreshment break to be taken at a convenient point 14a Terminal units function, identity and operational problems 14b AVSUs, LVA’s and Alarms operation and faults 14c Cylinder Management identification, storage, tracking, and connections 16:30 - 17:15 15 Moisture in medical gas systems 17.15 17.15 – 17.45 17.45 End of day two (industrial delegates) Manchester University session End of day two Course dinner at the Village Hotel (optional) 19.30 Paul Jones QC (MGPS) & Consultant (Medical Gases) Theresa Hughes Course Director Andrew Sully Cardiff and Vale NHS Trust Ed Doyle Altec (Analytical & Technical) Services Ltd Paul Jones QC (MGPS) & Consultant (Medical Gases) Keith Butler Alpha Moisture Systems Day Three – Wednesday 11 February 2015 Time 09:00 - 09:45 Session 16 Introduction to ‘permit to work’ system 09:45 - 10:45 17 10:45 - 11:15 11:15 - 12:00 12:00 - 13:00 13:00 – 17:30 17:30 - 17:45 17.45 Workshop ‘permit to work’ system Role-play workshop Refreshment Break 18 Introduction to pharmaceutical testing of gases How things are tested How instrumentation does its job Lunch 19 Workshop Sessions 5 parallel groups in rotation each workshop taking 30mins 19a OXYGEN identity and purity methods Speakers Mark Milne BOC Healthcare Mark Milne BOC Healthcare Ian Buckingham QC (MGPS) Quality Control North East Andrew Sully Cardiff and Vale NHS Trust 19b Ed Doyle NITROUS OXIDE / ENTONOX identity and purity methods Altec (Analytical & Technical) Services Ltd 19c Ian Buckingham MEDICAL, SURGICAL and DENTAL AIR identity and purity methods QC (MGPS) Quality Control North East 19d Paul Jones HELIOX and Surgical Carbon Dioxide identity and purity methods QC (MGPS) & Consultant (Medical Gases) 19e Adrian Fairbrother QC(MGPS) CONTAMINANTS moisture, particulates, and oil Refreshment Breaks to be taken at suitable times during workshop sessions Theresa Hughes Notices Course Director End of day three 2 Day Four – Thursday 12 February 2015 Time 09.00 – 09.30 Session 20 Calibration Gases When used etc Speakers Ian Buckingham QC (MGPS) Quality Control North East 09.30 – 10.00 21 Adrian Fairbrother QC(MGPS) 10.00 – 10.30 10.30 – 10.45 10.45 – 11.15 11.15 – 12.00 22 The Basic Tool Kit Refreshment Break 23 Common problems that occur with equipment 24 Measurements of particulates (Practical) 12.00 – 13.00 13:00 – 17.00 Lunch 25 - 17.00 Calibration of Instruments Problem solving workshop Workbook 45 minute to do test and write report Lecturers to oversee but stay back Theresa Hughes Course Director Richard Sutherland, Omicron Projects Paul Jones Tim Sizer, Paul Jones, Theresa Hughes Refreshment break to be taken at convenient time during the practical sessions End of day four Day Five – Friday 13 February 2015 Time 09.00 – 09.45 Session 26 Oxygen conservation 09.45 – 10.30 27 10.30 – 10.45 10.45 – 11.45 12.30 – 13.00 Refreshment break 28 Incident pit – ‘what can go wrong with medical gases’ Recent hazards, incidents and developments 29 Medical Gases - A Prescriber's View and Future Trends Closing summary 13.00 – 13.45 Lunch, close of course & issue of certificates 11.45 – 12.30 Dealing with pressure – new MG testers Speakers Steve Connew Colchester Hospital University NHS Foundation Trust Alistair Ellis-Jones, North East Wales NHS Trust Paul Jones QC (MGPS) & Consultant (Medical Gases) Dr Matthew Jones, Royal London Hospital Theresa Hughes Course Director 3