AP CHEMISTRY: CHAPTER 5
advertisement

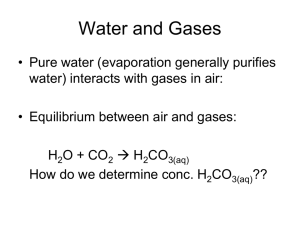
AP CHEMISTRY: CHAPTER 5 Gases In Chapter 5 you view the study of gases as fundamental to the development of the atomic theory of matter. You will study the bulk behavior of ideal gases (gas laws and their applications) and a microscopic model (Kinetic Molecular Theory). You will see that molecular weight and the speed of molecules can be determined using gas laws. Real gas deviations from the ideal gas laws are calculated. CA State Standards: 4a-h Concepts: 1. What is meant by the statement "the ideal gas law is an empirical equation"? 2. Why does your voice sound like Donald Duck after you inhale the gas from helium filled balloon? 3. What is assumed about gases which obey the Ideal Gas Law relationships? 4. Describe how a barometers and manometers are constructed. How do you use them to find the true pressure of an unknown gas? 5. Write the value of R in different sets of units common to gas pressure. If R can have so many different values, in what way is it a constant? 6. Density is usually measured in g/mL. Using "m" to stand for mass in grams, "V" to stand for volume in mL, and "D" for density, show how to rewrite the Ideal Gas Equation as a function of density. What value of R must be used to match the units indicated? 7. There are two reasons why real gases do not exactly obey the Ideal Gas Law. State these, and for each explain whether the observed volume is higher or lower than expected, and under what conditions each type of deviation is more noticeable. 8. Methane behaves in a distinctly different way from ideal gases as shown by Figure 5.25. Explain why the behavior difference is observed. 9. In the High Sierras back country, one would expect the rain water to be somewhat ________. Use chemical equations to explain the reason for this condition. Questions and Exercises: Chapter 5: 29,31,67,66,71,53,59,57,63,82,83,85,89,93,99,101 Lab Work: equipment) Experiment 7--Molar Mass of a Volatile Liquid (lab manual + handout for