ELECTROSTATIC COUPLING AND CONFORMATIONAL
FLUCTUATIONS AS DETERMINANTS OF PKA VALUES IN PROTEINS
by
Brian Doctrow
A dissertation submitted to Johns Hopkins University in conformity with the
requirements for the degree of Doctor of Philosophy
Baltimore, Maryland
March, 2014
© 2014 Brian Doctrow
All Rights Reserved
Abstract
Electrostatic effects, particularly proton binding and transfer, govern many essential
biological functions of proteins. Relating protein structure to function therefore
requires understanding the molecular determinants of pKa values in proteins.
Various factors influence these pKa values, including hydration, hydrogen bonding,
and Coulomb interactions. Resolving the contributions of these factors requires
structure-based calculations of electrostatic energies.
To be useful, such
calculations must be able to reproduce experimental data, which current structurebased pKa calculations are unable to do. This work examined two problems where
experimental insight was necessary to improve structure-based electrostatics
calculations.
Enzyme active sites typically contain clusters of ionizable residues, leading to
strong electrostatic interactions and complex coupling between the pKa values of the
residues involved. To better characterize these interactions, the ionizable residues
clustered in the active site of staphylococcal nuclease (SNase) were systematically
neutralized by mutagenesis, and the effect on the pKa values of the other ionizable
groups was measured using NMR spectroscopy. One of the residues in the active
site, Asp-19, has a depressed intrinsic pKa due to accepting a hydrogen bond, and is
therefore insensitive to repulsive Coulomb interactions. Meanwhile, Asp-21 has an
elevated intrinsic pKa due to acting as a hydrogen bond donor and therefore absorbs
most of the repulsive interaction energy in the cluster. Therefore in systems with
strong coupling between ionizable groups, small structural variations can lead to
ii
large differences in pKa values. Crystal structures may not be sufficiently accurate to
capture these variations.
It is believed that one reason for the failure of structure-based pKa
calculations is that they do not explicitly include the effects of backbone
reorganization. To show that backbone reorganization has a significant effect on
pKa values, the pKa values of carboxylic groups in SNase were measured in the
presence of glycine substitutions that perturbed the local stability of the protein
backbone.
Significant changes in pKa values were observed that could not be
reproduced with calculations that treat the protein backbone as static.
This
suggests that structure-based electrostatics calculations need to account for
backbone reorganization explicitly.
Thesis Committee
Bertrand García-Moreno E., Ph.D. (Advisor, Reader)
Juliette Lecomte, Ph.D. (Second Reader)
Mario Amzel, Ph.D.
Doug Barrick, Ph.D.
Vincent Hilser, Ph.D.
iii
Acknowledgements
First and foremost, thanks to Dr. Bertrand García-Moreno for his support and
encouragement throughout my graduate training. I am also grateful to my fellow
BGME lab members, past and present, for their assistance and companionship. I
especially want to thank Dr. Carlos Castañeda (for teaching me more about NMR
than I ever thought I’d learn), Dr. Carolyn Fitch (for explaining calculations and for
bringing doughnuts), Dr. Jamie Schlessman (for helping me navigate the harrowing
path of X-ray crystallography), Dr. Mike Harms (for showing me what computers
can do in the right hands), Dr. Aaron Robinson (for keeping the place nerdy), Erika
Wheeler (for always being happy and for watching my cat while I was out of town),
and Dan Richman (for many stimulating discussions).
Second, thanks to Dr. Ananya Majumdar, the NMR facility director. Much of
this work could not have been done without his commitment and support. I would
also like to thank the members of my thesis committee who have guided me
throughout my research: Dr. Juliette Lecomte, Dr. Doug Barrick, Dr. Vince Hilser, and
Dr. Mario Amzel.
Third, I want to thank my friends and fellow graduate students who have
shared my experiences. I thank Matt Preimesberger for sharing my love of classic
rock and for joining me for many concerts and baseball games. I thank Jackson Buss
for helping me take time out from research to play golf. I thank Dr. Helen Jun for
starting a turducken tradition and for showing me that life after graduate school is
possible.
I thank Mike Lee-Thompson for many nights of trivia fun and for
iv
introducing me to many new beers. And thanks to Thuy Dao, for her excellent
cooking.
I am grateful to my parents for always supporting me no matter what.
Thanks for always being there when I didn’t know where else to turn. Thanks also
for periodically getting me away from the lab and taking me on vacation.
Finally, I want to thank Amber Hill. Meeting her was the best thing to happen
to me during grad school. I could not have made it through the last couple of years
without her support, encouragement, and love. I hope I can be as good to her as she
has been to me, and I hope we have many more years and experiences to share.
v
Table of Contents
Abstract
ii
Acknowledgements
iv
Table of Contents
vi
List of Tables
viii
List of Figures
ix
1
1
INTRODUCTION
1.1 Importance of protein electrostatics in biology
1.2 pKa values of ionizable groups in proteins
1.3 Physical model of the determinants of pKa values of ionizable groups in proteins
1.4 Measurement of pKa values by NMR spectroscopy
1.5 Structure-based pKa calculations
1.6 pKa values of His, Asp, and Glu in staphylococcal nuclease
1.7 Overview of the contents of this dissertation
2
3
4
9
13
23
24
2 ELECTROSTATIC COUPLING IN A CLUSTER OF CARBOXYLIC GROUPS IN THE
ACTIVE SITE OF AN ENZYME
31
2.1 Abstract
2.2 Introduction
2.3 Results
2.3.1 Coulomb interactions in the active site cluster
2.3.2 pKa of Asp-21
2.3.3 pKa values at high ionic strength
2.3.4 Influence of Arg-35
2.4 Discussion
2.4.1 Determinants of the intrinsic pKa of Asp-21
2.4.2 Role of intrinsic binding affinities in partitioning of cooperative energy
2.4.3 Implications for structure-based pKa calculations
2.5 Conclusions
2.6 Materials and methods
2.6.1 Protein expression and purification
2.6.2 NMR spectroscopy
2.6.3 pKa values
2.6.4 Comparison of SNase structures
2.6.5 Crystal structure of ∆+PHS/D21N
2.6.6 Structure-based continuum electrostatic calculations
2.7 References
32
33
38
38
42
45
47
56
56
57
62
70
71
72
72
73
75
76
77
79
3 CONFORMATIONAL REORGANIZATION OF THE BACKBONE INFLUENCES THE PKA
VALUES OF IONIZABLE GROUPS IN PROTEINS
82
3.1 Abstract
83
vi
3.2 Introduction
3.3.1 pKa values measured by NMR spectroscopy
3.3.2 Thermodynamic stability
3.3.3 Crystal structures
3.3.4 Hydrogen exchange in Gly variants
3.3.5 15N NMR relaxation measurements
3.3.6 Structure-based pKa calculations
3.3.7 COREX calculations
3.4 Discussion
3.5 Conclusion
3.6 Materials and methods
3.6.1 Site directed mutagenesis and protein purification
3.6.2 Equilibrium thermodynamics
3.6.3 NMR spectroscopy
3.6.4 X-ray crystallography
3.6.5 Calculations
3.7 References
84
87
94
97
99
102
102
105
108
114
115
115
116
117
120
122
124
APPENDIX A SUPPLEMENTARY INFORMATION FOR CHAPTER 2, “ELECTROSTATIC
COUPLING IN A CLUSTER OF CARBOXYLIC GROUPS IN THE ACTIVE SITE OF AN
ENZYME”
130
A.1 References
146
APPENDIX B SUPPLEMENTARY INFORMATION FOR CHAPTER 3,
“CONFORMATIONAL REORGANIZATION OF THE BACKBONE INFLUENCES THE PKA
VALUES OF IONIZABLE GROUPS IN PROTEINS”
147
Vita
161
vii
List of Tables
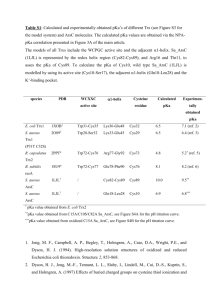
Table 2.1. pKa values of Asp and Glu residues in or near the active site of SNase measured at
100 mM KCl
39
Table 2.2. pKa values of Asp and Glu residues in or near the active site of SNase in 1M KCl. 46
Table 2.3. List of expected NOE interactions involving Arg-35-Hε for both the NVIAGA and
∆+PHS crystal structures.
53
Table 3.1. pKa values of select Asp and Glu residues measured by NMR spectroscopy.a
90
Table 3.2: Stability measured by acid- and GdmCl-induced denaturation.
95
Table A.1. pKa values of all Asp and Glu residues in all SNase variants from this study
measured at 100 mM KCl
131
Table A.2. pKa values for all carboxylic groups in ∆+PHS and ∆+PHS/D19N/D40N/E43Q
measured at 1 M KCl
140
Table A.3. X-Ray data collection and refinement statistics for ∆+PHS/D21N
142
Table B.1: pKa values of select Asp & Glu residues measured by NMR spectroscopy.a
148
Table B.2: Crystallographic statistics for ∆+PHS/M98G and ∆+PHS/A69G
153
Table B.3: RMSD of Gly variant crystal structures relative to ∆+PHS
155
Table B.4: Hydrogen exchange rates measured in ∆+PHS and Gly variants.a
156
viii
List of Figures
Figure 1.1. Examples of titration curves of Asp residues measured by NMR spectroscopy 11
Figure 2.1. Structures of the active sites of ∆+PHS and NVIAGA SNase variants
34
Figure 2.2. Asp-21 titration curves in the presence of charge-removal mutations
43
Figure 2.3. Titration curves for active site groups in ∆+PHS and R35Q
48
Figure 2.4. Histograms of distances from Arg-35 to Asp-19 or Asp-21 in SNase crystal
structures
51
Figure 2.5. NOEs involving Arg-35-Hε
54
Figure 2.6. Simulated titration curves for two interacting carboxylic groups
59
Figure 2.7. Effect of Asp-19 and Asp-21 on each other’s pKa
61
Figure 2.8. FDPB calculations for active site carboxylic groups
65
Figure 2.9. pKa values calculated during MD trajectories
68
Figure 3.1. Locations of Gly substitutions in SNase
88
Figure 3.2. pKa shifts caused by Gly substitutions in SNase
89
Figure 3.3. Effects of Gly substitutions on global stability
96
Figure 3.4. Alignment of Cα traces of ∆+PHS SNase and Gly variants
98
Figure 3.5. HX changes due to A69G and M98G substitutions
100
Figure 3.6. Backbone 15N relaxation parameters in ∆+PHS and Gly variants
103
Figure 3.7. Correlation between measured and calculated pKa values and shifts
104
Figure 3.8. Changes in COREX folding and protection constants due to Gly substitutions 106
Figure A.1. Structure of the active site of ∆+PHS/D21N
144
Figure B.1. pKa shifts in M98A and double Gly variants
160
ix
1 Introduction
1
1.1 Importance of protein electrostatics in biology
Many of the essential biological functions of proteins involve the transfer of
charge (e.g. protons (H+), electrons (e-), or ions such as Na+, K+, Ca2+, Mg2+, Cl-, etc.)
either between different compartments in a cell, between protein and solvent,
between protein and another molecule, or between different sites within the
protein. Examples of such functions include processes central to biological energy
transduction, such as H+ transport1–3 and e- transfer,4 ion homeostasis,5 and
catalysis.6 Because the energy of charge transfer depends on the electrostatic
potential difference between the start and end points, the ability of proteins to
perform charge transfer functions is governed by those properties that govern
electrostatic effects.
Electrostatic interactions also govern the pH-dependence of biochemical
processes.
For example, the pH-dependence of the equilibrium properties of
proteins arises from the differential proton affinities of different conformational
states of the protein. Classic examples of pH-dependent biological processes include
the modulation of the affinity of hemoglobin for oxygen7 and of the assembly of
many virus capsids8 by pH. Since protons (H+) are charged, the relevant differences
in binding affinity involve differences in electrostatic interactions between the
charged species of weak acids and bases and the different conformations of the
protein.
For all of these interesting biological processes, detailed physical
understanding of the relationship between structure and function requires knowing
the magnitude of electrostatic effects and understanding the factors that determine
2
them. It is well recognized that electrostatic energy is singularly valuable for
correlation of structure and function in biochemical processes in general.9
1.2 pKa values of ionizable groups in proteins
In proteins, the binding, release, and transfer of H+ involve primarily the
weak acids and bases of the ionizable moieties of Lys, His, Arg, Asp, and Glu. The
energetics of H+ binding and release are described by the pKa values of these groups,
which describe the equilibrium between the neutral and charged species of the
ionizable group. The pKa of an ionizable residue in water describes the energetic
balance between the proton-side chain bond and the proton-water bond. This is a
complicated balance, governed partly by quantum mechanical effects.
Since a change in the protonation state of an ionizable group involves a
change in charge state, the pKa of an ionizable residue in a protein will also be
influenced by the electrostatic properties of its milieu. Specifically, it will depend on
the electrostatic potential at the binding site, which is a complex function
determined by the geometry of the charges from other ionizable groups, by the
influence of permanent dipoles, and by the dielectric properties of the protein,
which are different from those of water. In general, the pKa can be expressed in
terms of the group’s pKa in a model compound in water plus the difference in
electrostatic energy between the protein and model compound states:10
model
pK a,i pK a,i
zi
Gelec,i
2.303RT
(1.1)
3
The energetics of e- transfer are described by redox potentials, which reflect
a similar equilibrium between charge states.
Therefore the same electrostatic
properties that influence pKa values also influence redox potentials. At many levels
and for many important problems in biochemistry, the problem of relating protein
structure to functions governed by electrostatics involves understanding the
molecular determinants of the pKa values (redox potentials) of the ionizable
residues (redox centers) involved.
1.3 Physical model of the determinants of pKa values of ionizable groups in
proteins
The pKa value of an ionizable group i is a measure of the Gibbs free energy
required to protonate (or deprotonate) that group. Invoking the additivity of the
Gibbs free energy function, the pKa values can be parsed into contributions from
different physical factors according to the following scheme:11,12
(1.2)
The term pKmodel refers to the pKa value of the group in a model compound in water.
This is a term that is meant to be determined empirically. It cannot be calculated
with precision because, as mentioned previously, it is a thermodynamic parameter
that is governed by a complicated balance between the energetics of H+ binding to
water versus the weak acid or weak base, which involves quantum effects. These
calculations are beyond even the most sophisticated quantum mechanical
4
calculations, primarily owing to uncertainties about the nature of the H+ in its
interaction with water. These pKmodel values have been experimentally determined
by a variety of approaches and under a variety of conditions. At 298 K, 0.1 M ionic
strength using peptides of various lengths with blocked N- and C-termini as model
compounds, the pKmodel values are 3.9, 4.4, and 6.5 for Asp, Glu, and His,
respectively,13 10.4 for Lys,14 12.0 for Arg, 10.0 for Tyr, and 9.0 for Cys.15
1.3.1 Hydration
The hydration of charged species is one of the strongest forces in biology. An
ionizable side chain in the charged state in bulk water is considered to be fully
hydrated and this hydration is reflected in the pKmodel values. In the protein, even at
the protein-water interface, the ionizable groups can be partially dehydrated. The
Born term, ∆pKBorn reflects the difference in the hydration energy of the charged
form of the group in water and in the protein interior. In a primitive continuum
electrostatics model the Born free energy can be described in terms of the free
energy for transferring a unit charge of radius r (in Å) between water and the
protein:
GBorn
332q2 1
1
2r in w
(1.3)
Here εin and εw are the dielectric constants of the protein and water, respectively.
The factor 332 converts the value of ∆G into units of kcal/mol. The free energy of
5
ionization is related to the pKa according to ∆G = 1.36*pKa (at 298K with ∆G in
kcal/mol). Because the protein interior is usually less polar and less polarizable
than water, εin will always be smaller than εw, and hence ∆∆GBorn will always be
unfavorable for the ionizable group in the protein relative to the ionizable group in
water. This will shift the pKa in the direction that favors the neutral state.
1.3.2 Coulomb Interactions
An ionizable group in a protein can experience two types of Coulomb effects.
The ∆pKbackground term in equation 1.2 reflects interactions between the ionizable
group and permanent dipoles within the protein. ∆pKij reflects interactions with
other charged ionizable groups. In a primitive continuum model with atomic detail
permanent dipoles are modeled as partial charges,11 so these two types of
interactions both follow Coulomb’s law:
Gij
332qiq j
rij
(1.4)
where qi and qj are the charges on groups i and j, and rij is the distance in Å between
groups i and j. Because the charged states of ionizable groups vary with pH, ∆pKij is
pH-dependent; the other terms in equation 1.2 are not.
The sum of the pH-
independent terms (pKmodel, ∆pKBorn, ∆pKbackground) is referred to as the intrinsic pKa
(pKint). It represents the pKa that the group would have if all of the other ionizable
groups in the protein were neutral. Save for Coulomb interactions with the charges
6
of other ionizable groups, pKint includes all effects on the pKa related to the ionizable
group being in a protein environment as opposed to bulk water.
1.3.3 pKa values in proteins are useful to examine the accuracy of structure-based
calculations
pKa values in proteins can be measured using NMR spectroscopy.16,17
Therefore, in principle, by comparing pKa values measured in proteins and pKa
values measured in model compounds, it is possible to determine the magnitudes of
electrostatic energies in proteins.
The pKa values of ionizable groups within proteins can vary considerably.
Groups at the protein surface tend to have pKa values similar to those of model
compounds.17,18 On the other hand, ionizable groups buried in the interior of a
protein can have highly anomalous pKa values quite different from those of model
compounds. The reason for this is that the dielectric effect inside a protein is much
smaller than that of water; therefore the Born energies can be very large and
uncompensated by background or Coulomb effects. For example, both Glu and Lys
have been substituted systematically at 25 internal positions in staphylococcal
nuclease. At 23 of these positions, Glu has a significantly elevated pKa compared to
its model compound value of 4.4.19
Similarly, the pKa of Lys is significantly
depressed at 19 of these positions compared to its model compound value of 10.4. 20
In both cases, the pKa values range from 5.2-9.4, corresponding to shifts of 1-5 pH
units from the corresponding model compound values.
7
One of the problems with attempting to understand the physical and
structural origins of electrostatic effects is that pKa measurements alone cannot
identify how the different terms in equation 1.2 contribute to a pKa. What is desired
is a correlation between the electrostatic energy and how the protein conformation
and dynamics are affected by a change in the charge of one group. The similarity
between pKa values of surface residues and the pKa values of model compounds may
indicate lack of interactions with the protein, or strong favorable interactions that
are canceled out by equally strong unfavorable interactions. To distinguish between
these two possibilities, structure-based pKa calculations with methods based on
physical principles are needed. With these methods one could attempt to calculate
the Born, background, and Coulomb contributions to pKa values starting from the
protein structure and principles from classical electrostatics and statistical
thermodynamics. For such calculations to be useful, they must be able to reproduce
experimental data to prove that they capture all of the relevant factors contributing
to the pKa.10 Current methods for structure-based pKa calculations (described in
section 1.4) are not able to reproduce experimental data well enough to have
predictive power.21 This suggests that our understanding of the physics governing
electrostatics in proteins is incomplete.
The development of more accurate
computational methods for structure-based calculations of electrostatic effects
remains one of the important goals in the area of structural biochemistry. The
experiments described in this dissertation examine two problems where
experiments are needed to obtain the detailed physical insight needed to guide the
8
development and improvement of accurate methods for structure-based calculation
of electrostatic energies.
1.4 Measurement of pKa values by NMR spectroscopy
The most useful way of accessing information about electrostatic effects in
proteins is by measurement of pKa values with NMR spectroscopy. Often a single
NMR spectroscopy experiment is sufficient to measure the pKa values of all the
ionizable groups of a given type simultaneously, sometimes unambiguously.16,17
The work described in this dissertation uses the pKa values of Asp and Glu residues
measured by NMR as probes of electrostatic properties of a protein.
For Asp and Glu, the carboxyl carbon (Cγ or Cδ) resonance is the best
reporter of the group’s protonation state for two reasons: (1) it exhibits large
changes in chemical shift upon protonation (typically 3-4 ppm);22,23 and (2) its
chemical shift is relatively insensitive to spurious pH-dependent effects that may
complicate the titration curves.22,23 For surface carboxyl residues, the carboxyl
carbon chemical shifts typically fall in the range of 175-180 ppm for Asp and 180185 ppm for Glu. In proteins with a large number of carboxylic groups, these
chemical shifts can be measured with a two-dimensional experiment that correlates
the carboxyl carbon (Cγ or Cδ) chemical shift with that of the neighboring aliphatic
carbon (Cβ or Cγ). This allows a large number of Asp & Glu resonances to be
resolved. For most residues, the carboxyl carbon resonance follows the titration of
only that residue, and the pH-dependence of the chemical shift has a characteristic
sigmoid shape, with the midpoint of the curve corresponding to the apparent pKa
9
value (Figure 1.1(a)). Apparent pKa values can be obtained by fitting a modified Hill
equation to the data:
obspH
AH A 10 n pHpK
a
(1.5)
110 npHpK a
where δAH and δA- are the chemical shifts of the fully protonated and fully
deprotonated forms, respectively, and n is the Hill coefficient, which reflects the
slope of the titration curve. Using this method, the pKa values for all Asp & Glu
residues in a protein can be measured simultaneously. The pKa value obtained in
this manner is an apparent pKa that is independent of pH, as opposed to the
microscopic pKa of equation 1.2 which is pH-dependent, as explained in section
1.3.2. The apparent pKa corresponds to the point where pKa,i(pH) = pH.
In certain cases, the titration curve does not follow the characteristic sigmoid
shape described by equation 1.5.
Such complexity may arise from strong
electrostatic interactions between two carboxylic groups that cause their
resonances to report on each other’s titration as well as their own. In these cases,
the modified Hill equation can be generalized to three-state binding to fit the data
better (Figure 1.1(b)).
Equation 1.5 assumes that proton binding and release occur in the fast
exchange regime, so that δobs is a weighted average of the chemical shifts of the
protonated and deprotonated states. In some cases severe line broadening occurs
10
Figure 1.1. Examples of titration curves of Asp residues measured by NMR
spectroscopy. (a) Example of a single-site titration curve and the fit line to equation
1.5. (b) Example of a curve that exhibits two titration events, and the fit to a three
state version of equation 1.5. (c) Example of a residue that titrates below the pH
where the protein unfolds, therefore no titration event is visible. In this case, the
pKa cannot be determined.
11
during the titration (see Figure 1.1(b)), indicating that the fast exchange condition is
no longer met. This is more likely to occur for residues with higher pKa values,
which titrate at lower [H+], and thus have slower exchange between protonation
states (kex = kon[H+] + koff). In such cases the pKa values determined using equation
1.5 will be less accurate. The degree of inaccuracy will depend primarily on how far
outside of the fast exchange regime the protonation rate is, which depends upon
both the exchange rate and the chemical shift difference between the protonated
and deprotonated species.24,25
Although NMR-monitored pH titrations provide an accurate way to measure
multiple pKa values within a protein, they are not without limitations. Chief among
these is that the protein has to remain folded during the titration of the residue(s) of
interest. In an unfolded protein, most residues’ resonances cannot be resolved
because the residues have lost their distinct chemical environments. Even if a
residue could still be resolved in the unfolded state, it will still be in a different
electrostatic environment from the one it experiences in the folded protein. Thus
the resulting titration curve will reflect a different pKa from the one the residue
would have in the folded state. This means that for Asp & Glu residues the protein
should remain folded at acidic pH (< 4), and even then the H+ titration curve of
residues with significantly depressed pKa values may not be measureable (Figure
1.1(c)). Therefore, proteins that fold only within a narrow pH range will not be
amenable to these types of measurements.
Furthermore, the limitation of
experimental pKa values mentioned in the previous section applies even to pKa
12
values measured with exquisite accuracy and precision by NMR spectroscopy: the
NMR experiments yield little direct insight into the determinants of the pKa values.
1.5 Structure-based pKa calculations
One of the goals of studying protein electrostatics is to understand how
structure determines pKa values. In fact, one of the goals of this thesis is to test two
specific hypotheses with the aim of contributing both the physical insight needed to
guide the development of computational algorithms for structure-based pKa
calculations, and the data necessary to benchmark these methods.
Various methods exist for calculating pKa values in proteins based on
structure and physical principles. These methods differ in the amount of atomic
detail that is treated explicitly, and in whether or not the protein structure is treated
as static or dynamic. At the most extreme level of detail are microscopic models, in
which all of the protein and solvent atoms and their motions are treated explicitly.
Such a model can provide the most rigorous insight into the physical origins of pKa
values. Unfortunately, these methods suffer from practical problems that make
them generally unsuitable for pKa calculations. These problems include difficulty
converging, improper treatment of long-range interactions, and artifacts resulting
from the treatment of the system boundary.10,26 Models based on the continuum
approximation are more useful. In these models, the polarizability of protein and
solvent are treated implicitly by assigning dielectric constants to these regions.
Electrostatic energies are then scaled according to these constants (see the
equations in section 1.3).
Different continuum models vary in how much
13
microscopic detail of the protein is retained (e.g. whether or not protein dipoles are
treated explicitly). In addition, some models account for protein motions explicitly
whereas others do not.
The use of dielectric constants greatly simplifies pKa
calculations. However, it also obscures the physical basis for the calculated pKa
values, since the dielectric constant subsumes a variety of processes that can
influence the pKa value.
1.5.1 FDPB calculations
One of the most popular methods for pKa calculations is based on the
numerical solution of the linearized Poisson-Boltzmann equation by the method of
finite-differences (FDPB).11 In this model, the protein is represented as a set of
stationary charges embedded in a medium with a uniform dielectric constant, εin.
Partial charges are used to represent permanent dipoles, which are assumed to have
a fixed orientation.
In the simplest FDPB implementation, the protein is
represented by a single, static structure. The solvent is represented as a continuous
medium with the dielectric constant of water, εw, and the concentrations of mobile
ions in solution are assumed to follow a Boltzmann distribution around the protein.
Solution of the Poisson-Boltzmann equation yields the electrostatic potential, Φij, at
site j due to a unit positive test charge at site i. Electrostatic energy is calculated as
the product of charge times potential, therefore the free energy of ionizing a group
at site i in the protein can be calculated using an expression such as:10
14
(1.6)
where the index j runs over all of the partial charges in the protein, and the index k
runs over all of the ionizable sites. The superscript ° designates the neutral state of
the group at i. The first two terms correspond to ∆pKBorn, the middle two to
∆pKbackground, and the last two to ∆pKij. An analogous expression can be used to
calculate the ionization energy in the model compound, ∆Gimodel. The pKa can then be
calculated as:
(1.7)
Implementation of FDPB calculations requires a number of parameters to be
specified by the user. First is a set of atomic coordinates, including H atoms (which
must be added computationally if their positions are not known experimentally). A
set of partial atomic charges must be provided, as well as the values of pKamodel for
each residue type. The temperature and ionic strength must be specified. Finally,
the values of the dielectric constants εw and εin must be assigned. εw is generally
assigned the value of the measured dielectric constant for bulk water (εw = 78.5).
The appropriate value of εin, on the other hand, is a matter of considerable debate.
Experimental measurements of the dielectric constant of dry protein powders, εprot,
give values in the range of 2-4.27 This value is comparable across many different
15
types of proteins, and is consistent with theoretical considerations.28 However,
using εin = εprot in standard FDPB calculations exaggerates the magnitude of
electrostatic effects. Ionizable groups at the surface of the protein tend to have
measured pKa values close to their model compound values,17,18 but the calculations
with εin = 4 predict pKa values that are considerably shifted from their model
compound pKa values. Agreement between calculated and measured pKa values of
surface residues can be improved by setting εin = 20, thereby artificially attenuating
electrostatic interactions.29
The reason that FDPB calculations with static structures fail when εin = εprot
lies in the fact that the physical meaning of these two parameters is not the same.
The measured parameter εprot reflects a fundamental property of proteins, namely,
the bulk dielectric response of the protein molecules to an external electric field. By
contrast, what determines the pKa values of ionizable residues is the dielectric
response of a single protein molecule to a charge within that molecule. In this sense,
εin is not a true dielectric constant, rather it is a scaling parameter meant to account
for all contributions to electrostatic interactions that the protein model does not
treat explicitly.26,30 Its physical meaning depends entirely on the way the protein is
modeled in the calculations. In a fully microscopic simulation, all contributions to
electrostatic interactions are treated explicitly, therefore εin = 1.
If electronic
polarizability is not treated explicitly, then εin has to account for its effect implicitly,
resulting in εin ≈ 2. As the protein model gets less detailed, more effects get
subsumed into εin, causing the value of εin to increase further. Since a standard FDPB
calculation represents the protein with a static structure, it does not account for
16
dynamic contributions to electrostatic effects explicitly. These can range from
fluctuations of charged side chains and reorientation of dipoles to large-scale
structural reorganization and even global unfolding in the most extreme cases. 26
Supposedly εin = 20 accounts implicitly for the effects of these dynamics on the pKa
values of surface residues.29
Unfortunately, the ability to reproduce the pKa values of surface residues
alone is insufficient to show that a calculation captures the correct physical
determinants of pKa values. As noted in section 1.3, surface groups tend to have pKa
values similar to those of model compounds. Any calculation with a high εin will
predict weak interactions between residues and consequently, small shifts from
model compound pKas,26 even if these small shifts do not actually reflect weak
interactions. Internal ionizable residues, whose pKa values are very different from
model compounds, provide a more stringent test for identifying physically realistic
models. Although using εin = 20 in FDPB calculations with static structures can give
reasonable results for surface ionizable residues,29 the pKa values of internal
ionizable residues are better reproduced using εin = 10,30 which does not reproduce
the pKa values of surface residues. Thus a protein model that treats dynamic effects
implicitly via εin is a poor model, because there is no value of εin that can selfconsistently reproduce the pKa values of surface and internal ionizable groups
simultaneously. This reflects the fact that the protein interior is a heterogeneous
environment. Different atoms within the protein will not be equally polarizable, and
there is no compelling reason to assume that all parts of the protein will exhibit the
same structural and dynamic response to ionization. Therefore the same value of εin
17
may not be valid for all ionizable groups and there is no way to know a priori what
value to use for any given group.10 In order for structure-based calculations to selfconsistently reproduce the pKa values of all ionizable residues simultaneously,
structural reorganization must be treated explicitly.
1.5.2 Structural reorganization by molecular dynamics
One way to model structural reorganization explicitly is by using molecular
dynamics (MD) simulations.
In the simplest implementation, an ensemble of
conformations is generated from an MD simulation, and an average pKa is computed
from the ensemble. Early calculations of this sort on bacteriorhodopsin31 and
cytochrome c32 showed that averaging had a significant effect on the calculated pKa
values. However, it was difficult to judge whether the conformational averaging
improved the accuracy over calculations with static structures because
experimental pKa values for most residues in these proteins were either unavailable
or poorly determined. The first study to compare pKa values calculated from MD
ensembles with measured pKa values was by van Vlijmen et al.33 These authors
calculated pKa values in BPTI and lysozyme from MD-generated ensembles using
three different approaches to conformational averaging.
They also performed
single-structure calculations using two different crystal structures of each protein.
They found that for both proteins, pKa values calculated from MD ensembles were as
good or better than calculations from a single crystal structure, depending on which
crystal structure was used. However, even with conformational averaging, the
calculated pKa values were more accurate using εin = 20 versus εin = 4.
18
The limitations of using MD simulations to account for conformational
reorganization have been discussed previously. Bashford and Gerwert pointed out
the inability of classical MD to account for conformational changes linked to
titration.31
If a residue is assumed to be in the charged state (the standard
protonation state at pH 7 for Arg, Lys, Asp, and Glu), then the MD simulation will be
biased towards conformations that stabilize the charged state.
Consequently,
favorable Coulomb interactions will be exaggerated and the pKa value will be shifted
too far in the direction favoring the charged state. Use of εin = 20 may compensate
for this bias by attenuating favorable interactions, resulting in the improved
accuracy seen by van Vlijmen et al.33 One way to include coupled titration and
conformational reorganization is to run two simulations, one with the residue of
interest protonated and one with the residue deprotonated, and then average the
results of the two simulations using a linear response approximation.26
For
calculations of the pKa values of all ionizable residues in a protein, this becomes
problematic because the large number of possible protonation states requires
generating an equally large number of MD trajectories. Another difficulty with MD
simulations is that they are limited in the range of timescales that can be sampled.
Large changes, such as local unfolding or water penetration, may have a substantial
impact on pKa values yet cannot be sampled adequately in the timescales accessible
to current MD simulations.21,34
Consequently, a high value of εin may still be
necessary to account for the effects of processes that are slow compared to the
timescale of the simulations.
19
1.5.3 Constant-pH molecular dynamics
Recently, constant-pH molecular dynamics (CPHMD) methods have been
developed to address some of the aforementioned issues with classical MD. In these
methods, coupling between conformational dynamics and protonation is explicitly
modeled using one of two approaches: (1) continuous titration coordinates are
propagated alongside the spatial coordinates using λ dynamics,35 or (2) discrete
protonation states are sampled throughout the simulation via Monte Carlo.36 In
such an approach, while explicit solvent can be used in calculation of the
conformational state, it is not practical to calculate protonation states using explicit
solvent because of the lengthy simulation times required to compute solvation
forces accurately.35 Instead, most of these methods calculate protonation states
using a generalized Born implicit solvent model. Unfortunately, this model is known
to underestimate effective Born radii for buried atoms, which leads to
overestimation
of
solvation
energies
and
underestimation
of
Coulomb
interactions.37 CPHMD methods also suffer from problems with slow convergence,37
although the introduction of enhanced sampling techniques such as replica
exchange35 and accelerated MD38 may alleviate some of these problems.
1.5.3 Multi-conformation continuum electrostatics
Another method that accounts for conformational reorganization explicitly is
the multi-conformation continuum electrostatics (MCCE) method.39,40
In this
method, each side chain in the protein is allowed to adopt multiple rotameric and
tautomeric states (conformers) with energies calculated using a continuum
20
approach.
The populations of the conformers at specific pH values are then
determined using Monte Carlo sampling. Thus the coupling between structural
reorganization and ionization is treated explicitly for side chains, as well as for sitebound ions and internal water molecules. MCCE improves the accuracy of pKa
calculations when εin lower than 20 is used, illustrating just how sensitive the
calculated pKa values can be to small changes in the local microenvironment.40
However, arbitrary adjustments to εin are still necessary to get the best accuracy.41
Comparison of calculations on different crystal structures of the same protein give
different results, and pKa values averaged over multiple crystal structures tend to be
more accurate than values calculated from a single structure.40 Furthermore, MCCE
does a poor job of reproducing the pKa values of residues whose ionization is
coupled to unfolding.42 These results imply that backbone reorganization, which is
not treated explicitly in the MCCE calculations, can have a significant impact on pKa
values.
1.5.4 Other ensemble-modulated continuum electrostatics
Backbone reorganization can be treated explicitly with the ensemblemodulated continuum electrostatics (EMCE) method.43 This method is based on the
COREX algorithm,44,45 which generates a Boltzmann-weighted ensemble of partially
unfolded microstates from a single input structure.
Within each microstate,
ionizable groups that are in folded regions of the protein and sufficiently protected
from solvent are assigned microscopic pKa values calculated from a single structure
with a continuum method. Groups in unfolded regions or that are exposed to
21
solvent are assigned model compound pKa values. The protonation state of each
residue is then averaged over the entire ensemble at each pH to obtain titration
curves. Each residue’s overall pKa will then be a population-weighted average:
pK a Pf pK a, f Pu pK a,u
(1.8)
where pKa,f and pKa,u are the pKa values in the folded and unfolded states,
respectively, and Pf and Pu are the populations of those states. Residues that are in
less stable regions of the protein, and thus more prone to local unfolding, will have
more normal pKa values in the ensemble calculations compared with the static
structure calculations. Furthermore, because groups can have different pKa values
in different microstates, the populations of the microstates will be pH-dependent.
Specifically, as the pH decreases, microstates with higher pKa values will become
more favorable. This method has been shown to reproduce correctly the acidunfolding behavior of staphylococcal nuclease,43 but its ability to reproduce
individual pKa values has yet to be tested. This method also ignores the effects of
side chain reorganization within folded regions of the protein. Thus the EMCE and
MCCE techniques are complementary to each other. MCCE only treats side chain
reorganization explicitly, while EMCE only treats local & global unfolding explicitly.
However, neither technique on its own provides a complete description of the
protein response to ionization.
22
1.6 pKa values of His, Asp, and Glu in staphylococcal nuclease
The studies described in the following chapters used staphylococcal nuclease
(SNase) as a model system for probing electrostatic effects in proteins in detail.
SNase is extremely useful as a model protein owing to its relatively small size (149
residues), its high solubility that makes it highly amenable to experimental analysis
in general and to NMR studies in particular, its high stability, and the ease with
which it can be crystallized and manipulated.
It contains a large number of
ionizable residues: 23 Lys, 12 Glu, 8 Asp, 5 Arg, and 4 His, which provides the
possibility of many electrostatic interactions at the protein surface. The pKa values
of all 20 Asp & Glu residues have been measured previously by NMR,46 as have those
of His residues.47,48 FDPB calculations can reproduce the pKa values of the His
residues,48, but not those of the Asp and Glu residues,46 even when the protein is
treated with εin = εw. The only time reasonable agreement between calculated and
measured pKa values of Asp and Glu residues is obtained is when the calculations
are carried out using 1 M ionic strength.
This suggests that the calculations
overestimate the magnitude of the ∆pKij term in equation 1.2, since raising the ionic
strength increases screening of medium- and long-range Coulomb interactions. For
carboxylic residues, these interactions are predominantly attractive, thus
overestimation of these interactions leads to calculated pKa values that are too
depressed. This leads the calculations to overestimate the number of protons taken
up during unfolding and to predict the protein to unfold at a much higher pH than is
observed experimentally.21
Furthermore, these calculations are unable to
reproduce the anomalous pKa values of the active site residues. Thus SNase is useful
23
as a model system in which to demonstrate the limitations of structure-based
electrostatics calculations.
Studying the determinants of pKa values in SNase
experimentally can provide insight into why these calculations fail, and hopefully
lead to improvements in the calculations as well as our general understanding of the
relationship between protein structure and electrostatic energy.
1.7 Overview of the contents of this dissertation
The studies described in this dissertation examine two situations in which
pKa values are particularly difficult for structure-based electrostatics calculations to
reproduce. The first is when multiple ionizable groups come together to form a
cluster, such as might be found in an enzyme active site. The second is when
ionizable residues are under the influence of significant backbone conformational
reorganization.
To understand why the calculations fail in these instances,
experimental data that provide physical insight into the determinants of the pKa
values are needed. Measuring how pKa values in a protein shift in response to
specific mutations can provide this insight, if we also know how those mutations
affect other physical properties of the protein. The resulting data can be used to
evaluate the physical accuracy of existing methods for structure-based pKa
calculations, as well as to guide the development of more accurate methods.
Clusters of ionizable residues are typically found in the active sites of
enzymes, where ionizable residues facilitate catalysis by acting as general
acids/bases or as nucleophiles, or by stabilizing transition states through
electrostatic interactions.6,49 Having multiple ionizable residues in close proximity
24
creates strong electrostatic interactions, which give rise to highly shifted pKa values
and complex titration curves for the clustered residues.49–51 Because of these
interactions, the pKa values of the clustered residues are strongly coupled to each
other, and reflect a precise balance between the different interactions represented
by the terms in equation 1.2. Small changes to any one of these interactions can
affect all of the residues in the cluster significantly. Consequently the pKa values in
such a cluster are sensitive to small variations in structure, making structure-based
pKa values extremely difficult. To our knowledge, nobody has yet attempted to
dissect the interactions in such a cluster experimentally.
Chapter 2 of this dissertation comprises a detailed characterization of
electrostatic interactions in the active site of SNase.
The active site of SNase
contains a cluster of four carboxylic groups with very different pKa values. One pKa
is elevated, one is depressed, and the other two are unchanged relative to model
compound pKa values. FDPB calculations consistently fail to reproduce these pKa
shifts. To understand why participation in the cluster has such different effects on
each group’s pKa, the ionizable residues in and around this cluster were
systematically neutralized by mutagenesis, and the pKa values in these variants
were measured by NMR spectroscopy. This enabled dissection of the detailed
network of interactions present in this cluster and to separate intrinsic (pKint) from
cooperative (pKij) contributions to each residue’s pKa.
Chapter 3 explores the hypothesis that backbone reorganization influences
pKa values. As outlined in section 1.5, the high values of εin required for continuum
calculations to reproduce the pKa values of surface groups are believed to account
25
implicitly for the effects of conformational reorganization.29
In some cases,
however, even using εin = εw is not sufficient to reproduce experimental pKa values.
Such is the case with the Asp and Glu residues in SNase. 46 This implies that the
single protein structure used in the calculations is an inadequate representation of
the protein in solution, which exists in an ensemble of conformations. Support for
this view comes from calculations using the EMCE method described in section 1.5,
which are better at reproducing the acid unfolding of SNase than FDPB calculations
with a single structure.43
The success of the EMCE model, which treats
conformational reorganization as a local unfolding process, suggests that pKa values
are coupled to local conformational stability. This hypothesis was tested using
amino acid substitutions intended to perturb the local stability of the protein
backbone without affecting the overall, global structure. Changes to the measured
pKa values in these variants are strong evidence that local stability has a significant
influence on pKa values and must be treated explicitly in electrostatics calculations.
The data from this chapter also provide a useful test of the accuracy of methods that
explicitly model conformational reorganization.
26
1.7 References
1. Burykin, A. & Warshel, A. (2003). What Really Prevents Proton Transport through
Aquaporin? Charge Self-Energy versus Proton Wire Proposals. Biophysical Journal
85, 3696–3706
2. Burykin, A. & Warshel, A. (2004). On the origin of the electrostatic barrier for proton
transport in aquaporin. FEBS Letters 570, 41–46
3. Braun-Sand, S., Strajbl, M. & Warshel, A. (2004). Studies of Proton Translocations
in Biological Systems: Simulating Proton Transport in Carbonic Anhydrase by EVBBased Models. Biophysical Journal 87, 2221–2239
4. Gunner, M.R. & Alexov, E. (2000). A pragmatic approach to structure based
calculation of coupled proton and electron transfer in proteins. Biochimica et
Biophysica Acta (BBA) - Bioenergetics 1458, 63–87
5. Burykin, A., Kato, M. & Warshel, A. (2003). Exploring the origin of the ion
selectivity of the KcsA potassium channel. Proteins: Structure, Function, and
Bioinformatics 52, 412–426
6. Warshel, A. (2003). COMPUTER SIMULATIONS OF ENZYME CATALYSIS:
Methods, Progress, and Insights. Annual Review of Biophysics and Biomolecular
Structure 32, 425–443
7. Chu, A.H., Turner, B.W. & Ackers, G.K. (1984). Effects of protons on the
oxygenation-linked subunit assembly in human hemoglobin. Biochemistry 23, 604–
617
8. Ehrlich, L.S., Liu, T., Scarlata, S., Chu, B. & Carter, C.A. (2001). HIV-1 Capsid
Protein Forms Spherical (Immature-Like) and Tubular (Mature-Like) Particles in
Vitro: Structure Switching by pH-induced Conformational Changes. Biophysical
Journal 81, 586–594
9. Warshel, A. & Levitt, M. (1976). Theoretical studies of enzymic reactions: Dielectric,
electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme.
Journal of Molecular Biology 103, 227–249
10. García-Moreno E, B. & Fitch, C.A. (2004). Structural Interpretation of pH and SaltDependent Processes in Proteins with Computational Methods. Methods in
Enzymology Volume 380, 20–51
11. Bashford, D. & Karplus, M. (1990). pKa’s of ionizable groups in proteins: atomic
detail from a continuum electrostatic model. Biochemistry 29, 10219–10225
12. Warshel, A. (1981). Calculations of enzymic reactions: calculations of pKa, proton
transfer reactions, and general acid catalysis reactions in enzymes. Biochemistry 20,
3167–3177
13. Castaneda, C.A. (2009). Determinants of electrostatic energies and pKa values in
proteins. at <http://search.proquest.com/docview/304907798?accountid=11752>
14. Keim, P., Vigna, R.A., Nigen, A.M., Morrow, J.S. & Gurd, F.R.N. (1974). Carbon 13
Nuclear Magnetic Resonance of Pentapeptides of Glycine Containing Central
Residues of Methionine, Proline, Arginine, and Lysine. J. Biol. Chem. 249, 4149–
4156
15. Matthew, J.B., Gurd, F.R.N., Garcia-Moreno, B.E., Flanagan, M.A., March, K.L. &
Shire, S.J. (1985). pH-Dependent Processes in Protein. Critical Reviews in
Biochemistry and Molecular Biology 18, 91–197
27
16. Markley, J.L. (1975). Observation of histidine residues in proteins by nuclear
magnetic resonance spectroscopy. Accounts of Chemical Research 8, 70–80
17. Forsyth, W.R., Antosiewicz, J.M. & Robertson, A.D. (2002). Empirical relationships
between protein structure and carboxyl pKa values in proteins. Proteins: Structure,
Function, and Genetics 48, 388–403
18. Edgcomb, S.P. & Murphy, K.P. (2002). Variability in the pKa of histidine side-chains
correlates with burial within proteins. Proteins: Structure, Function, and
Bioinformatics 49, 1–6
19. Isom, D.G., Castañeda, C.A., Cannon, B.R., Velu, P.D. & García-Moreno E., B.
(2010). Charges in the hydrophobic interior of proteins. Proceedings of the National
Academy of Sciences of the United States of America 107, 16096 –16100
20. Isom, D.G., Castañeda, C.A., Cannon, B.R. & E, B.G.-M. (2011). Large shifts in pKa
values of lysine residues buried inside a protein. Proceedings of the National
Academy of Sciences of the United States of America 108, 5260–5265
21. Fitch, C.A., Whitten, S.T., Hilser, V.J. & García‐ Moreno E., B. (2006). Molecular
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
mechanisms of pH‐ driven conformational transitions of proteins: Insights from
continuum electrostatics calculations of acid unfolding. Proteins: Structure,
Function, and Bioinformatics 63, 113–126
Oda, Y., Yamazaki, T., Nagayama, K., Kanaya, S., Kuroda, Y. & Nakamura, H.
(1994). Individual Ionization Constants of All the Carboxyl Groups in Ribonuclease
HI from Escherichia coli Determined by NMR. Biochemistry 33, 5275–5284
Chen, H.A., Pfuhl, M., McAlister, M.S.B. & Driscoll, P.C. (2000). Determination of
pKa Values of Carboxyl Groups in the N-Terminal Domain of Rat CD2: Anomalous
pKa of a Glutamate on the Ligand-Binding Surface. Biochemistry 39, 6814–6824
Feeney, J., Batchelor, J.G., Albrand, J.P. & Roberts, G.C.K. (1979). The effects of
intermediate exchange processes on the estimation of equilibrium constants by NMR.
Journal of Magnetic Resonance (1969) 33, 519–529
Sudmeier, J.L., Evelhoch, J.L. & Jonsson, N.B.-H. (1980). Dependence of NMR
lineshape analysis upon chemical rates and mechanisms: Implications for enzyme
histidine titrations. Journal of Magnetic Resonance (1969) 40, 377–390
Schutz, C.N. & Warshel, A. (2001). What are the dielectric “constants” of proteins
and how to validate electrostatic models? Proteins: Structure, Function, and
Bioinformatics 44, 400–417
Takashima, S. & Schwan, H.P. (1965). Dielectric Dispersion of Crystalline Powders
of Amino Acids, Peptides, and Proteins1. The Journal of Physical Chemistry 69,
4176–4182
Gilson, M.K. & Honig, B.H. (1986). The dielectric constant of a folded protein.
Biopolymers 25, 2097–2119
Antosiewicz, J., McCammon, J.A. & Gilson, M.K. (1994). Prediction of Phdependent Properties of Proteins. Journal of Molecular Biology 238, 415–436
Fitch, C.A., Karp, D.A., Lee, K.K., Stites, W.E., Lattman, E.E. & García-Moreno,
E.B. (2002). Experimental pKa Values of Buried Residues: Analysis with Continuum
Methods and Role of Water Penetration. Biophysical Journal 82, 3289–3304
Bashford, D. & Gerwert, K. (1992). Electrostatic calculations of the pKa values of
ionizable groups in bacteriorhodopsin. Journal of Molecular Biology 224, 473–486
28
32. Zhou, H.-X. & Vijayakumar, M. (1997). Modeling of protein conformational
fluctuations in pKa predictions. Journal of Molecular Biology 267, 1002–1011
33. Van Vlijmen, H.W.T., Schaefer, M. & Karplus, M. (1998). Improving the accuracy
of protein pKa calculations: Conformational averaging versus the average structure.
Proteins: Structure, Function, and Bioinformatics 33, 145–158
34. Warshel, A., Sharma, P.K., Kato, M. & Parson, W.W. (2006). Modeling electrostatic
effects in proteins. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics
1764, 1647–1676
35. Wallace, J.A. & Shen, J.K. (2011). Continuous Constant pH Molecular Dynamics in
Explicit Solvent with pH-Based Replica Exchange. Journal of Chemical Theory and
Computation 7, 2617–2629
36. Mongan, J., Case, D.A. & McCammon, J.A. (2004). Constant pH molecular
dynamics in generalized Born implicit solvent. Journal of Computational Chemistry
25, 2038–2048
37. Alexov, E., Mehler, E.L., Baker, N., M. Baptista, A., Huang, Y., Milletti, F., Erik
Nielsen, J., Farrell, D., Carstensen, T., Olsson, M.H.M., Shen, J.K., Warwicker, J.,
Williams, S. & Word, J.M. (2011). Progress in the prediction of pKa values in
proteins. Proteins: Structure, Function, and Bioinformatics 79, 3260–3275
38. Williams, S.L., de Oliveira, C.A.F. & McCammon, J.A. (2010). Coupling Constant
pH Molecular Dynamics with Accelerated Molecular Dynamics. Journal of Chemical
Theory and Computation 6, 560–568
39. Alexov, E.G. & Gunner, M.R. (1997). Incorporating protein conformational
flexibility into the calculation of pH-dependent protein properties. Biophysical
Journal 72, 2075–2093
40. Georgescu, R.E., Alexov, E.G. & Gunner, M.R. (2002). Combining conformational
flexibility and continuum electrostatics for calculating pKas in proteins. Biophysical
Journal 83, 1731–1748
41. Gunner, M.R., Zhu, X. & Klein, M.C. (2011). MCCE analysis of the pKas of
introduced buried acids and bases in staphylococcal nuclease. Proteins: Structure,
Function, and Bioinformatics 79, 3306–3319
42. Song, Y., Mao, J. & Gunner, M.R. (2009). MCCE2: Improving protein pKa
calculations with extensive side chain rotamer sampling. Journal of Computational
Chemistry 30, 2231–2247
43. Whitten, S.T., García-Moreno E., B. & Hilser, V.J. (2005). Local conformational
fluctuations can modulate the coupling between proton binding and global structural
transitions in proteins. Proceedings of the National Academy of Sciences of the
United States of America 102, 4282–4287
44. Hilser, V.J. & Freire, E. (1996). Structure-based Calculation of the Equilibrium
Folding Pathway of Proteins. Correlation with Hydrogen Exchange Protection
Factors. Journal of Molecular Biology 262, 756–772
45. Hilser, V.J. & Freire, E. (1997). Predicting the equilibrium protein folding pathway:
Structure-based analysis of staphylococcal nuclease. Proteins: Structure, Function,
and Bioinformatics 27, 171–183
46. Castañeda, C.A., Fitch, C.A., Majumdar, A., Khangulov, V., Schlessman, J.L. &
García‐ Moreno, B.E. (2009). Molecular determinants of the pKa values of Asp and
29
47.
48.
49.
50.
51.
Glu residues in staphylococcal nuclease. Proteins: Structure, Function, and
Bioinformatics 77, 570–588
Lee, K.K., Fitch, C.A., Lecomte, J.T.J. & García-Moreno E, B. (2002). Electrostatic
Effects in Highly Charged Proteins: Salt Sensitivity of pKa Values of Histidines in
Staphylococcal Nuclease†. Biochemistry 41, 5656–5667
Lee, K.K., Fitch, C.A. & García-Moreno E., B. (2002). Distance dependence and salt
sensitivity of pairwise, coulombic interactions in a protein. Protein Science 11, 1004–
1016
Søndergaard, C.R., McIntosh, L.P., Pollastri, G. & Nielsen, J.E. (2008).
Determination of electrostatic interaction energies and protonation state populations
in enzyme active sites. Journal of Molecular Biology 376, 269–287
McIntosh, L.P., Hand, G., Johnson, P.E., Joshi, M.D., Körner, M., Plesniak, L.A.,
Ziser, L., Wakarchuk, W.W. & Withers, S.G. (1996). The pKa of the general
acid/base carboxyl group of a glycosidase cycles during catalysis: a 13C-NMR study
of bacillus circulans xylanase. Biochemistry 35, 9958–9966
Ondrechen, M.J., Clifton, J.G. & Ringe, D. (2001). THEMATICS: A simple
computational predictor of enzyme function from structure. Proceedings of the
National Academy of Sciences of the United States of America 98, 12473–12478
30
2 Electrostatic Coupling in a Cluster
of Carboxylic Groups in the Active
Site of an Enzyme
(to be submitted in a slightly different form to Journal of Molecular Biology under
the authorship of Brian M. Doctrow, Carlos A. Castañeda, Carolyn A. Fitch, Ananya
Majumdar, Maja Cieplak, Jamie L. Schlessman, and Bertrand García-Moreno E.)
31
2.1 Abstract
Clusters of ionizable groups in active sites of enzymes are useful to examine
the partitioning of Gibbs free energy in cooperative and allosteric ligand binding
systems. The active site cluster of staphylococcal nuclease consists of two carboxylic
groups (Asp-40, Glu-43) with near normal pKa values (3.9 and 4.3, respectively), one
(Asp-19) with a low value of 2.2, and one (Asp-21) with an anomalous value of 6.5.
Typical of active sites and other such clusters, FDPB calculations cannot reproduce
the measured pKa values using εin = 20. NMR spectroscopy was used to examine the
partitioning of cooperative interaction free energy between these four H+ binding
sites. H+ titration curves of all carboxylic groups were measured for variants in
which the charge of the cluster was modified. The data suggest that Asp-19 is
insensitive to repulsive Coulomb interactions because it has a low intrinsic pKa
value, a consequence of accepting a hydrogen bond from the backbone amide of
Asp-21 and of favorable Coulomb interactions not observed in the crystal structure.
Asp-21 absorbs most of the repulsive interaction energy in the cluster because it has
an elevated intrinsic pKa, the result of its acting as a hydrogen bond donor to the
backbone carbonyl of Val-39.
This cluster of carboxylic groups exhibits the
amplification of small perturbations that is the hallmark of cooperative systems.
These results illustrate problems inherent to structure-based calculations of ligand
binding energy in cooperative ligand binding systems, where small and unavoidable
inaccuracies of the models used in the calculations and of the crystal structures used
by the models are amplified, thereby compromising the accuracy of the calculations.
Implications of these findings for structure-based pKa calculations are discussed.
32
2.2 Introduction
Biological function in many proteins results from energetically coupling
processes occurring in different parts of the molecule. Electrostatic forces govern
biological function in enzymes, proton pumps, viruses, and other systems where
cooperative H+ binding interactions are essential.
Enzymes are especially
noteworthy in this respect, as they often have clusters of ionizable groups at their
active sites, and strong Coulomb interactions and structural reorganization coupled
to ionization events can lead to anomalous pKa values and complex titration
curves.1,2 The active site of xylanase, for example, contains two acidic residues: Glu78, which titrates with a near normal pKa of 4.6, and Glu-172, which titrates with an
anomalous pKa of 6.7.3 Understanding how coupling free energy can be distributed
in a network of ligand binding sites,4 and specifically how the pKa values of
individual H+ binding sites are affected by cooperative interactions within clusters
of ionizable groups is essential to understanding the relationship between protein
structure and function.
The active site of staphylococcal nuclease (SNase), with a cluster of four
carboxylic residues (Asp-19, Asp-21, Asp-40, and Glu-43), is well suited for in-depth
studies of coupling energies in charged clusters (Figure 2.1(a)). Previous studies5–7
have shown that all of these residues except Asp-19 are crucial for catalysis. The
distribution of pKa values in this cluster is striking: Asp-21 has a pKa = 6.5, Asp-19
has a pKa = 2.2, and Asp-40 and Glu-43 have normal values of 3.9 and 4.3,
respectively.8 The molecular determinants of these pKa values are not obvious in
33
Figure 2.1. (a) Structure of the active site of ∆+PHS SNase at 1.80 Å (PDB accession
code: 3BDC)8 showing the side chains of Asp-19, Asp-21, Arg-35, Asp-40, and Glu43, as labeled. Shortest distances between the ionizable moieties are indicated. Also
shown in purple is an apparent hydrogen bond between Asp-21 and the backbone
amide of Thr-41. The green sphere indicates where Ca2+ is bound. (b) Structure of
the active site the NVIAGA/E75A variant of SNase at 2.01 Å (PDB accession code
2RDF)9 showing the side chains of Asp-19, Asn-21, Arg-35, and Asp-40, as labeled
(Glu-43 is disordered). Shortest distances from Arg-35 to Asp-19 or Asn-21 are
indicated. Also shown in purple is the apparent hydrogen bond between Asn-21
and the backbone carbonyl of Val-39.
34
SNase crystal structures, which appear not to reflect the conformational state of this
region of the protein in solution.
The carboxylic groups in this cluster are known to interact with each other.
For example, titration curves obtained from the pH dependence of the chemical
shifts the Cγ and Hβ of Asp-19 measured with NMR spectroscopy report on the
titration of Asp-21, and vice versa.8 Similarly, the Cγ and Hβ chemical shifts of Asp40 also appear to report on the protonation state of Asp-21. Furthermore, Asp-40
and Glu-43 have low Hill coefficients, even in the presence of 1M KCl, suggesting
that their titration is under the influence of other groups with comparable pKa.8 The
pKa values suggest that the free energy from cooperative interactions is not
partitioned evenly among the cluster elements, and that Asp-21 absorbs all of the
repulsive interactions in the cluster. Crystal structures do not provide insight into
the structural and physical origins of this apparent asymmetry.
The H+ titration curves described by the pH-dependence of chemical shifts
measured with NMR spectroscopy represent individual-site binding isotherms.4
They describe the free energy for binding H+ to one specific site while binding can
occur simultaneously at all other sites.
In charged systems the cooperative
interactions between ligand binding events at different individual H + binding sites
can be mediated directly through Coulomb interactions, and indirectly through
conformational changes that shift the equilibrium of the H+ binding site between
charged and neutral states. The individual-site binding isotherms thus represent a
convolution of numerous processes and interactions. In scenarios more complex
than a two- or three-site ligand binding system, it would be impossible to
35
deconvolute analytically the intrinsic, microscopic ligand binding constants for each
site from the cooperative interaction energies.4 However, because the intrinsic
ligand binding properties of the different ionizable sites can be studied
independently by measurement of pKa values of model compounds in water (e.g. pKa
= 4.0 or 4.5 for Asp or Glu in water, respectively), it is possible to measure
cooperative interactions in individual-site binding isotherms empirically.
Ackers and co-workers demonstrated analytically that the free energy of
cooperative interactions in a two-site ligand binding system need not be distributed
symmetrically.4 Instead the cooperative interaction will have a larger effect on the
binding site with the weaker intrinsic binding affinity. In the case of two carboxylic
groups with cooperative interactions mediated by Coulomb forces, the largest share
of the cooperative interaction will be allocated to the site with the higher intrinsic
pKa, defined as the pKa that the group would have in the absence of other ionizable
groups. When the intrinsic pKa values of the two sites are very different, the one
with the higher intrinsic pKa will be neutral in the pH range where the site with the
lower intrinsic pKa titrates. Therefore the site with low intrinsic pKa titrates as if the
other site is not there.
Figure 2.1(a) depicts the active site of SNase as observed in atomic
coordinates (PDB accession code 3BDC)8 that can be considered representative of
published structures of SNase. The apparent hydrogen bond between Asp-21 and
the backbone amide of Thr-41 should lower the pKa of Asp-21. The inferred ion pair
between Asp-21 and Arg-35 should compensate for repulsive Coulomb interactions
with other members of the cluster and maybe also lower its pKa. Instead, the pKa of
36
Asp-21 is 6.5, significantly higher than the Asp model compound pKa of 4.0. Burial
of a carboxyl oxygen could lead to an elevated pKa because dehydration will
destabilize the charged state. However the average solvent accessibility of the
carboxyl oxygen atoms of Asp-21 is similar to that of Asp-19 (16% vs. 19%), so the
shift in pKa resulting from dehydration is unlikely to be an issue. It is not obvious
from the crystal structure why the pKa of Asp-21 is high and that of Asp-19 is low.
This project sought to examine the nature of cooperative interactions in the
charge cluster. Charges in or near the cluster were removed systematically with
site-directed mutagenesis, and H+ binding isotherms for all carboxylic groups in the
variants were measured with NMR spectroscopy.8 The crystal structure of SNase
variant ∆+PHS/D21N was determined, and the conformations of the active site
cluster in 119 crystal structures of SNase were compared. Nuclear Overhauser
effects (NOEs) were used to examine specific atomic contacts in the active site
region of the protein in solution.
After the interactions in the cluster were
understood with sufficient detail to explain why the pKa values of Asp-19 and Asp21 are so different, pKa values were calculated with a variety of continuum
electrostatics methods to illustrate the inherent difficulties with structure-based
calculation of pKa values in clusters of ionizable groups where strong cooperative
interactions are present. In these cases, precisely because of the cooperative nature
of the system, the accuracy of energy calculations is compromised by the
unavoidable amplification either of inaccuracies inherent to the electrostatic models
or of artifacts in the crystal structures.
37
2.3 Results
2.3.1 Coulomb interactions in the active site cluster
To determine the contributions from pairwise Coulomb interactions to the
pKa values of carboxylic groups in the active site cluster, variants of the highly stable
∆+PHS form of SNase were created in which the ionizable groups in the cluster
(Asp-19, Asp-21, Arg-35, Asp-40, and Glu-43) were replaced with the non-ionizable
analogues Asn or Gln. The ∆+PHS form of SNase was used as the background
protein to ensure it remained folded in the pH range where Asp and Glu residues
titrate, so that their pKa values could be measured.
Of the 8 Asp and 12 Glu residues in SNase, most were insensitive to these
substitutions and exhibited no shifts in pKa (Appendix A). The D19N, D40N, and
E43Q substitutions lowered pKa values in the cluster, consistent with removing
repulsive Coulomb interactions. Significant repulsive interactions were apparent
between Asp-19 and Asp-21, Asp-19 and Glu-43, Asp-40 and Glu-43, and Asp-21 and
Asp-40, but not between Asp-19 and Asp-40 (Table 2.1), which is not surprising
because Asp-19 and Asp-40 are on opposite ends of the cluster (Figure 2.1(a)) and
farther apart than any other pair of carboxylic groups in the cluster. Glu-43 also
appeared to interact with Glu-52, whose ionizable moiety is roughly 6 Å from that of
Glu-43, but which does not interact with any of the other groups in the cluster.
The pKa of Asp-19 increased slightly in the E43Q variant. Similarly, the D21N
substitution caused the pKa values of the other groups in the cluster to increase.
Neither effect can be explained in terms of Coulomb interactions, as the mutated
38
Table 2.1. pKa values of Asp and Glu residues in or near the active site of SNase measured at 100 mM KCl
Protein
∆+PHSc
∆+PHS/D19N
∆+PHS/D21N
∆+PHS/D40N
∆+PHS/E43Q
∆+PHS/D19N/D40N/E43Q
Residue
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
pKaa
2.12 ± 0.05d,e
6.54 ± 0.01e
3.83 ± 0.05e
4.32 ± 0.03
3.93 ± 0.05
5.75 ± 0.02e
3.80 ± 0.03
3.79 ± 0.01
3.85 ± 0.02
2.60 ± 0.01d
3.94 ± 0.01
4.46 ± 0.02
4.10 ± 0.03
2.19 ± 0.01d,e
6.18 ± 0.01e
4.11 ± 0.01
3.77 ± 0.02
2.34 ± 0.01d,e
6.16 ± 0.01e
3.69 ± 0.01e
3.65 ± 0.01
4.57 ± 0.01
39
∆pKab
-0.79 ± 0.02
-0.03 ± 0.06
-0.52 ± 0.03
-0.08 ± 0.05
0.48 ± 0.05
0.11 ± 0.05
0.14 ± 0.05
0.17 ± 0.06
0.07 ± 0.05
-0.36 ± 0.01
-0.21 ± 0.03
-0.16 ± 0.05
0.22 ± 0.05
-0.38 ± 0.01
-0.14 ± 0.05
-0.28 ± 0.05
-1.97 ± 0.01
na
0.81 ± 0.01d,e
1.03 ± 0.02e
0.65 ± 0.01e
0.69 ± 0.01
0.65 ± 0.02
0.94 ± 0.02e
0.56 ± 0.02
0.68 ± 0.01
0.69 ± 0.01
0.82 ± 0.02
0.68 ± 0.01
0.67 ± 0.02
0.66 ± 0.02
0.93 ± 0.02d,e
0.97 ± 0.01e
0.73 ± 0.01
0.75 ± 0.02
0.81 ± 0.03d,e
0.93 ± 0.01e
0.83 ± 0.01e
0.75 ± 0.01
0.93 ± 0.02
∆+PHS/R35Q
∆+PHS/D19N/R35Q/D40N/E43Q
∆+PHS/D21N/R35Q/D40N/E43Q
∆+PHS/R35Q/D40N/E43Q
PHS
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
Asp-40
Glu-43
Glu-52
3.55 ± 0.01
3.06 ± 0.01d,e
6.05 ± 0.01e
4.27 ± 0.01
4.45 ± 0.02
3.89 ± 0.02
4.65 ± 0.01
3.67 ± 0.01
3.46 ± 0.03
3.76 ± 0.02
3.10 ± 0.05e
5.70 ± 0.01
3.78 ± 0.01
2.05 ± 0.05d,e
6.12 ± 0.05
3.73 ± 0.02e
3.74 ± 0.03e
3.90 ± 0.02
40
-0.38 ± 0.05
0.94 ± 0.05
-0.49 ± 0.01
0.44 ± 0.05
0.13 ± 0.04
-0.04 ± 0.02
-1.89 ± 0.01
-0.26 ± 0.05
1.34 ± 0.06
-0.17 ± 0.05
0.98 ± 0.07
-0.84 ± 0.01
-0.15 ± 0.05
-0.07 ± 0.07
-0.42 ± 0.05
-0.10 ± 0.05
-0.58 ± 0.04
-0.03 ± 0.05
0.87 ± 0.02
0.88 ± 0.02d,e
0.89 ± 0.02e
0.64 ± 0.01
0.63 ± 0.02
0.61 ± 0.01
0.90 ± 0.02
0.85 ± 0.02
0.96 ± 0.04
0.86 ± 0.02
0.87 ± 0.08e
0.99 ± 0.02
0.91 ± 0.01
0.63 ± 0.06d,e
0.81 ± 0.06
0.84 ± 0.03e
0.76 ± 0.07e
0.79 ± 0.02
pKa values and Hill coefficients obtained by fitting the modified Hill equation (equation (2.2)) to the pH-dependence of the Cγ/Cδ chemical shift,
unless otherwise indicated. Titrations were performed at 298 K and 100 mM KCl. Values reported are those from a single titration experiment with
corresponding errors of fit, unless otherwise indicated.
b Change in pKa relative to ∆+PHS at 100 mM KCl: ∆pKa = pKavariant – pKa∆+PHS
c Values reported for ∆+PHS at 100 mM KCl are means and standard errors over 3 independent titration experiments, using the data from Castañeda et
al.8
d pKa and Hill coefficient determined by fixing the amplitude (∆δ) of the transition to the ∆δ obtained from the fit for the same residue in ∆+PHS at 1 M
KCl.
e pKa and Hill coefficient obtained by fitting a two-site model (equation (2.3)) to the pH-dependence of the Cγ/Cδ chemical shift. Only the values
corresponding to the larger of the two transitions are reported.
a
41
groups are neutral in the range where the affected carboxylic groups titrate. These
observations suggest the D21N and E43Q substitutions must somehow perturb
conformational equilibria in the cluster.
The cluster of carboxylic groups forms a binding site for Ca2+ and the crystal
structures of ∆+PHS and many other SNase variants show a site-bound Ca2+ ion
within this cluster. However, previous work has demonstrated that treating SNase
with EDTA does not affect the pKa values of the carboxylic groups measured with
NMR spectroscopy, suggesting that trace amounts of Ca2+ do not affect these pKa
values.8 pKa values of carboxylic groups in ∆+PHS were also measured when Ca2+
and the inhibitor thymine 3’-5’-diphosphate (pdTp) were added at the same time.
The resulting changes were minimal (< 0.2 pH units) for all residues except Asp-21,
whose pKa decreased to 5.36 (data not shown). In addition, certain residues showed
a second titration event having about the same pKa as Asp-21, which presumably
corresponds to the pH-dependent dissociation of Ca2+ and pdTp. Thus, it seems
likely that even when they are present in the sample, Ca2+ and pdTp are not bound
to a significant extent below pH 5, where most carboxylic groups titrate.
2.3.2 pKa of Asp-21
The titration curves of Asp-21 show that replacement of any carboxylic
group in the cluster with a neutral group lowers the pKa of Asp-21 relative to its
value of 6.54 ± 0.01 in the ∆+PHS protein (Figure 2.2(a)). This is fully consistent
with the presence of unfavorable Coulomb interactions between Asp-21 and all
other carboxylic groups in the cluster. Note that even when all three carboxylic
42
Figure 2.2: (a) Titration curves for Asp-21 in ∆+PHS SNase (black circles) and in the
D19N (cyan), D40N (red), E43Q (green), D19N/D40N/E43Q (orange), and
D19N/R35Q/D40N/E43Q (violet) variants, all at 0.1 M KCl. (b) Titration curves for
Asp-21 in ∆+PHS SNase (black) and in the D19N/D40N/E43Q variant (orange) at
0.1 M KCl (filled circles) and at 1 M KCl (open circles). Lines represent fits of a
modified Hill equation to the data. Except for D19N/D40N/E43Q and
D19N/R35Q/D40N/E43Q at 0.1 M KCl, a two-site modified Hill equation (equation
(2.3)) was used, where the Hill coefficient of the smaller transition was fixed
arbitrarily to be 1.0. This smaller transition reflects the titration of either Asp-19 (in
∆+PHS, D40N, and E43Q), Asp-40 (in D19N), or Asp-83 (in D19N/D40N/E43 at 1 M
KCl). A one-site modified Hill equation (equation (2.2)) was fitted to the data for
D19N/D40N/E43Q and D19N/R35Q/D40N/E43Q at 0.1 M KCl.
43
groups were replaced, the pKa of Asp-21 was 4.57, which is 0.67 pH units higher
than the normal pKa of 3.9 for Asp in water.8 The effects of these substitutions were
not additive: the ∆pKa of Asp-21 when all three carboxylic groups were replaced
was −1.97 ± 0.01 pH units, whereas the sum of the ∆pKa values for the single-residue
variants resulted in a net pKa shift of only −1.53 ± 0.02.
In ∆+PHS at 0.1 M KCl, the resonance of Asp-21 broadens significantly at
pHvalues near its pKa.8 Such broadening of Asp-21 was observed to some extent in
all of the variants, and probably reflects exchange between protonated and
deprotonated forms. Since the rate of this exchange is pH-dependent (kex = koff +
kon[H+]), a higher pKa corresponds to slower exchange during titration, possibly
resulting in intermediate as opposed to fast exchange.10 In variants where the pKa
of Asp-21 is lower than 6, the broadening is less pronounced, and the peak remains
visible throughout the titration (Figure 2.2), consistent with faster exchange at
lower pH.
Wild-type SNase contains a disordered loop spanning residues 44-49 that
is excised in ∆+PHS SNase. This loop is close to the active site and contains four
basic residues (Lys-45, His-46, Lys-48, and Lys-49). To test whether the pKa of Asp21 was affected by the presence of this loop, the pKa values of carboxylic groups
were also measured in the SNase variant PHS, in which the 44-49 loop is present
and which has Gly-50 and Val-51 as opposed to Phe-50 and Asn-51 in ∆+PHS.11 The
measured pKa of Asp-21 in PHS SNase was 6.12, which is 0.42 pH units lower than in
∆+PHS (Table 2.1). The pKa of Glu-43 in PHS SNase was 0.58 pH units lower than in
∆+PHS. These changes are consistent with the presence of favorable Coulomb
44
interactions between Asp-21 and the basic residues in the loop in the wild type
protein. However, even in the presence of these interactions, the pKa of Asp-21 is
still significantly higher than the pKa of Asp in water.
2.3.3 pKa values at high ionic strength
The pKa values of carboxylic groups in ∆+PHS were measured previously at
both 0.1 M and 1 M KCl to estimate the overall contribution of Coulomb interactions
to the pKa values of these groups.8 This assumes that because salt screens Coulomb
interactions, all contributions from Coulomb effects to pKa values are eliminated at
high salt. Upon increasing from 0.1 M to 1 M KCl, the pKa of Asp-21 decreases by
0.52 units, which is only 20% of the total difference between the Asp-21 pKa at 0.1 M
KCl (6.54 ± 0.01) and the normal pKa of Asp in water (3.90 ± 0.01) (Table 2.2 and
Figure 2.2(b)).8
The pKa of Asp-21 was elevated even when the other carboxylic groups in the
active site were removed (Table 2.1). To determine if this was caused by long-range
repulsive Coulomb interactions with residues outside of the active site, the pKa
values of Asp and Glu residues in the D19N/D40N/E43Q variant of ∆+PHS were
measured in 1 M KCl. For most residues, the differences between 0.1 and 1 M KCl
were comparable in the variant and in ∆+PHS (Appendix A). The pKa shift of Glu-52
due to increased KCl was more than twice as large in the variant as in the reference
protein (Table 2.2). This can be explained if one assumes that in the background
protein the simultaneous screening of repulsive Coulomb interactions offsets the
increase in pKa from screening of attractive Coulomb interactions. As noted earlier,
45
Table 2.2. pKa values of Asp and Glu residues in or near the active site of SNase in
1M KCl.
Protein
Residue
Asp-19
Asp-21
c
∆+PHS
Asp-40
Glu-43
Glu-52
Asp-19
Asp-21
∆+PHS/D19N/D40N/E43Q Asp-40
Glu-43
Glu-52
pKaa
2.88 ± 0.02d
6.02 ± 0.01d
4.28 ± 0.01
4.40 ± 0.01
4.08 ± 0.02
5.01 ± 0.01d
3.91 ± 0.01
∆pKab
0.76 ± 0.05
-0.52 ± 0.01
0.45 ± 0.05
0.08 ± 0.03
0.15 ± 0.05
0.44 ± 0.01
0.36 ± 0.01
na
0.83 ± 0.04d
0.94 ± 0.02d
0.81 ± 0.01
0.81 ± 0.02
0.84 ± 0.02
0.95 ± 0.02d
0.94 ± 0.02
pKa values and Hill coefficients obtained by fitting the modified Hill equation (equation ( 2.2)) to the
pH-dependence of the Cγ/Cδ chemical shift, unless otherwise indicated. Titrations were performed
at 298 K and 1 M KCl. Values reported are those from a single titration experiment with
corresponding errors of fit.
b Change in pKa relative to the same variant at 100 mM KCl: ∆pKa = pKa1M – pKa100mM
c pKa values obtained using the data from Castañeda et al.8
d pKa and Hill coefficient obtained by fitting a two-site model (equation ( 2.3)) to the pH-dependence
of the Cγ/Cδ chemical shift. Only the values corresponding to the larger of the two transitions are
reported.
a
46
the pKa values indicate a repulsive Coulomb interaction between Glu-43 and Glu-52.
In the D19N/D40N/E43Q variant this repulsive interaction has been removed,
leaving only attractive interactions to be screened by salt. This effect was even
more pronounced in the change in the pKa of Asp-21. Whereas in ∆+PHS increasing
the salt concentration lowered the pKa of Asp-21, in the D19N/D40N/E43Q variant
increasing the salt concentration elevated the pKa of Asp-21 to a value of 5.01 (Table
2.2 and Figure 2.2(b)). These results indicate that in the absence of Asp-19, Asp-40,
and Glu-43, the net Coulomb interactions sensed by Asp-21 are attractive, and that
Asp-21 is not under the influence of significant Coulomb interactions with
negatively charged groups outside the active-site region.
2.3.4 Influence of Arg-35
In the crystal structure of ∆+PHS SNase (Figure 2.1(a)), the carboxyl group of
Asp-21 is within 4 Å of the Arg-35 guanidino group, suggesting a favorable Coulomb
interaction between these two groups. To test this, pKa values were measured in the
R35Q variant (Table 2.1). Because this substitution removes an apparent favorable
Coulomb interaction with Asp-21, the pKa of Asp-21 was expected to shift up
relative to the background. However, the pKa of Asp-21 in the R35Q variant was
0.49 units lower than in the background (Figure 2.3). One possible explanation is
that Gln-35 could form a hydrogen bond to Asp-21 that compensates for the loss of
the favorable Coulomb interaction with Arg-35.
In the R35Q variant the pKa value of Asp-19 was elevated by 0.94 units
relative to the background, consistent with the loss of a strong Coulomb attraction
47
Figure 2.3. (following page) Titration curves for carboxylic groups in the active site
in both ∆+PHS (black) and the R35Q variant (red). Lines represent fits of equation
(2.2) or (2.3) to the data. Equation (2.2) was used to fit Asp-40 in the R35Q variant
and Glu-43 in both variants, whereas equation (2.3) was used to fit Asp-19 and Asp21 in both variants, and Asp-40 in ∆+PHS. Dashed lines indicate that the fit was
performed using a fixed value of ∆, as described in Materials and Methods.
48
49
between Asp-19 and Arg-35. This is surprising given that in the crystal structure,
these groups are relatively far apart (~7 Å) and both groups are exposed to bulk
water (Figure 2.1(a)). The pKa of Asp-40, which is closer (~5.5 Å) to Arg-35 in the
crystal structure, was elevated by only 0.44 units in the R35Q variant and that of
Glu-43 by only 0.13 units, entirely consistent with the large (~8 Å) distance between
Arg-35 and Glu-43. In the absence of the other carboxylic groups, Arg-35 has no
significant effect on the pKa of Asp-21 (Table 2.1 and Figure 2.2(a)).
To explain the apparent strong Coulomb interaction between Arg-35 and
Asp-19, the conformations observed for this region of the protein in 119 crystal
structures of SNase in the Protein Data Bank12 were compared. Histograms of the
distances between residues 19 and 35 (Figure 2.4(a)) and between 21 and 35
(Figure 2.4(b)) in 119 crystal structures are both bimodal. The two clusters in each
distribution correspond to cases with or without Ca2+ bound at the active site. In
nearly all cases Asp-19 is at least 7 Å away from Arg-35 and Asp-21 is always within
5 Å of Arg-35. However, two outliers (PDB accession codes 2QDB and 2RDF)9 were
also identified in which Asp-19 and Arg-35 are closer (< 6 Å). Both of these outliers
were variants of the NVIAGA form of SNase, so called after the six substitutions used
to make it (D21N, T33V, T41I, S59A, P117G, S128A).9 The D21N substitution
changes the hydrogen-bonding capacity of this residue. The crystal structure shows
Asn-21 replacing Arg-35 as a hydrogen-bonding partner to the backbone carbonyl
of residue 39 (Figure 2.1(b)). Arg-35 in turn adopts a conformation that puts it
closer to Asp-19. A similar conformation for Arg-35 has been observed in other
NVIAGA variants (Doctrow et al., in preparation).
50
This conformation is more
Figure 2.4. (a) Histogram of distances between the side chains of residues Asp-19
and Arg-35 in the SNase structures listed in Materials and Methods. The peaks of
the clusters that correspond to the presence and absence of Ca2+ are indicated. (b)
Same as (a) for the distances between residues Asp-21 and Arg-35.
51
consistent with the apparent strong Coulomb interaction between Asp-19 and Arg35 (Figure 2.3), suggesting that this conformation is more representative of the
structure of SNase in solution at the pH where Asp-19 titrates.
To better determine whether the crystal structure of ∆+PHS or NVIAGA
better represents the conformation of Arg-35 in solution at low pH, a
15N-NOESY-
HSQC spectrum was collected for ∆+PHS at pH 4.68. Table 2.3 lists all of the
expected NOE interactions involving Arg-35-Hε for both the NVIAGA and ∆+PHS
crystal structures using a cutoff distance of 4 Å. Many more NOEs are expected for
the NVIAGA conformation than for the ∆+PHS conformation, allowing the two
conformations to be distinguished in solution. All but one of the observed NOEs
involving Arg-35-Hε are consistent with the NVIAGA structure, but several of them
are inconsistent with the ∆+PHS crystal structure (Figure 2.5, colored peaks &
atoms). The observed NOE involving a Leu-36-Hδ is not consistent with either
structure. However, the distance between Arg-35-Hε and Leu-36-Hδ1, which is the
closer of the two leucine methyl groups, is much shorter in the NVIAGA structure
than in the ∆+PHS structure.
Taken together, these NOEs indicate that the
conformation of Arg-35 in solution at low pH is more similar to the NVIAGA crystal
structure than to the ∆+PHS structure, and the small remaining discrepancies may
be attributed to crystal packing interactions. Therefore, pKa calculations based
solely on the ∆+PHS crystal structure will not be correct because the crystal
structure does not reflect the relevant conformation in solution.
52
Table 2.3. List of expected NOE interactions involving Arg-35-Hε for both the
NVIAGA and ∆+PHS crystal structures.
Atoma
Thr-22-Hα
Thr-22-Hγ1
Thr-22-Hγ2
Arg-35-Hα
Arg-35-Hβ2
Arg-35-Hγ2
Arg-35-Hγ3
Arg-35-Hδ2
Arg-35-Hδ3
Leu-36-HN
Leu-36-Hδ1
Arg-87-Hδ2
Arg-87-HH12
Arg-87-HH22
Distance from Arg-35 (Å)
∆+PHSb
NVIAGAc
6.38
3.09
6.3
3.13
4.17
2.53
4.85
2.52
4.37
2.69
2.87
3.5
3.53
3.94
2.94
2.94
2.35
2.35
5.14
3.82
7.68
5.33
5.51
3.98
2.77
5.03
2.99
5.42
NOE expected ?
∆+PHS
NVIAGA
No
Yes
No
Yes
No
Yes
No
Yes
No
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
No
Yes
No
No
No
Yes
Yes
No
Yes
No
Hydrogen coordinates added to structures using the MolProbity server
(molprobity.biochem.duke.edu).13,14
b Distances obtained from the crystal structure of holo-∆+PHS (PDB accession code 3BDC)
c Distances obtained from the crystal structure of NVIAGA/E75Q (PDB accession code 2QDB).
a
53
Figure 2.5: (a) F2(1H)-F3(1H) slice from the 15N-NOESY-HSQC spectrum of ∆+PHS
centered on the Arg-35-Nε and Hε resonances in F1 and F3, respectively. NOESY
peaks that are not expected based on the ∆+PHS crystal structure are colored by
residue: green = Arg-35, cyan = Thr-22, magenta = Leu-36. Structure of the
environment of Arg-35 in (b) NVIAGA (PDB accession code 2QDB)9 and (c) ∆+PHS
(PDB accession code 3BDC).8 Atoms involved in the observed NOE interactions are
colored as in (a).
54
2.3.5 Crystal structure of the D21N variant
To determine if the presence of Asn-21 altered the conformation of Arg-35,
the crystal structure of ∆+PHS/D21N was determined. The conformation of Arg-35
is similar to that in ∆+PHS, and not to that in either of the NVIAGA structures
(Appendix A). However, the ∆+PHS/D21N crystal structure includes the bound
inhibitor pdTp, which is not present in the NVIAGA structures.
One of the
phosphate groups of this inhibitor interacts directly with Arg-35, and may serve to
stabilize the side chain and bias the conformation of the active site.
There are almost no intermolecular interactions in the ∆+PHS/D21N crystal
structure involving the cluster residues that could bias the conformation of these
residues.
No polar atoms from neighboring molecules come within hydrogen
bonding distance of residue 21. Residues Arg-105, Lys-127, and Lys-134 from a
neighboring molecule are all within 7 Å of Glu-43 and may therefore form favorable
Coulomb interactions with Glu-43, but not with any of the other cluster residues.
Similar interactions are observed in the ∆+PHS crystal structure. In addition, in the
∆+PHS/D21N crystal structure the N-terminus of a neighboring molecule is within 6
Å of residues 19, 35 and 43, and therefore there may be Coulomb interactions
between the N-terminus and these groups. The closest intermolecular Coulomb
contact is 4.5 Å, between Asp-19-Oδ2 and the N-terminus of a neighboring molecule.
The N-terminus is not ordered in most SNase crystal structures, including ∆+PHS.
The structure confirms that for the purposes of pKa calculations, crystal structures
alone may not reliably represent the state of the protein in solution. Certain states
55
may crystallize more easily than others, and those states may not correspond to the
dominant states in solution.
2.4 Discussion
2.4.1 Determinants of the intrinsic pKa of Asp-21
A protonated Asp-21 acting as a hydrogen bond donor should stabilize the
protonated state and lead to a high intrinsic pKa.15 In the NVIAGA crystal structures
Asn-21 replaces Arg-35 as a hydrogen bond donor to the backbone carbonyl of Val39, and this may be the reason for the difference in the conformation of Arg-35
between NVIAGA and other SNase variants. In variants with Asp at position 21, this
hydrogen bond could form only when Asp-21 is protonated under conditions of pH
lower than those under which the crystals were grown. If this hydrogen bond does
determine the conformation of Arg-35, this would render the conformations of Asp21 and Arg-35 pH-sensitive. Since the crystal structures of ∆+PHS and all other
SNase variants were obtained from crystals grown at a pH above the pKa of Asp-21,
the crystal structures are likely to show Arg-35 in the conformation present when
Asp-21 is charged. However, all of the other carboxylic groups in SNase titrate at pH
values well below that of Asp-21 and so their titrations will be influenced by Arg-35
in the conformation corresponding to Asp-21 neutral.8 This is supported by the
effect of the R35Q substitution on the pKa of Asp-19 (Figure 2.3), and by the NOEs
involving Arg-35-Nε (Figure 2.5). Both of these results are more consistent with the
conformation observed in the NVIAGA crystal structure than in the ∆+PHS crystal
56
structure. Chemical shifts of atoms up to 12 Å away from Asp-21 report on its
titration,8 which could indicate a conformational change linked to the titration of
Asp-21.
In contrast to Asp-21, the pKa of Asp-19 is depressed. Even when all other
ionizable groups in the active site (including Arg-35) are neutralized, Asp-19 has a
pKa of 3.46, which is 0.44 pH units lower than the normal pKa of 3.9 for Asp in
water.8 This suggests a depressed intrinsic pKa for Asp 19. This could result from
Asp-19 acting as a hydrogen bond acceptor, which would stabilize the deprotonated
state.
Inspection of the crystal structures of several nuclease variants reveals
multiple potential donors within hydrogen bonding distance of Asp-19.8 In addition,
the Asp-21 HN chemical shift appears to follow the titration of Asp-19, which
suggests a hydrogen bond between these two groups.8
2.4.2 Role of intrinsic binding affinities in partitioning of cooperative energy
For any given pair of ionizable residues A and B, the Coulomb interaction
between them will contribute to the pKa of A only when some fraction of B is in the
charged state while A is titrating, et vice versa. Thus the pKa of site A will depend on
the extent of binding at B, giving rise to a cooperative interaction between the two
binding sites and a pH-dependence for the Coulomb interaction. This situation is
described by the following thermodynamic cycle:
57
Here ∆GAint and ∆GBint refer to the intrinsic proton binding affinities of groups A and
B, respectively, and ∆GAB represents the energy of the cooperative (Coulomb)
interaction between them. Titration curves for the individual sites derived from this
cycle are shown in Figure 2.6. Dashed lines are simulated titration curves for two
non-interacting sites (∆GAB = 0) with different intrinsic pKa values. Solid lines are
the titration curves for the same groups when they are assumed to have a Coulomb
interaction between them. It is evident from the curves in Figure 2.6 that the
titration of the group with the lower intrinsic pKa (black) is largely unaffected by the
presence of the Coulomb interaction, whereas the titration of the group with the
higher intrinsic pKa (red) is shifted to a significantly higher pH. This is consistent
with the analysis of Ackers et al.4
Titration curves resembling the solid lines in Figure 2.6 have been observed
for residues in the active sites of various enzymes.1,3 These active sites tend to have
multiple ionizable groups carrying like charge in close proximity and protected from
solvent, resulting in strong Coulomb interactions.
In fact, titration curves
resembling those in Figure 2.6 have been used as diagnostics to identify ionizable
58
Fraction charged
1.0
0.8
0.6
0.4
0.2
0.0
0
2
4
6
8
10
pH
Figure 2.6. Simulated titration curves for two carboxylic groups in the absence of
any interaction (∆GAB = 0, dashed lines) and in the presence of a Coulomb
interaction between them (solid lines). The black lines correspond to a group with
intrinsic pKa of 3.8 and the red lines to an intrinsic pKa of 4.6. The energy of the
Coulomb interaction (∆GAB) has been arbitrarily set to 3.1 kcal/mol to emphasize
the fact that when there is an interaction it is the weaker of the two binding sites
that is most affected and therefore shifted the most.
59
groups in enzyme active sites.2
It has been demonstrated that such strong
cooperative interactions result in a free energy of protonation that is relatively
constant over a wide pH range. Such a mechanism may help catalytic residues to
retain the proper protonation state over a wider environmental pH range.16
The pKa of Asp-21 in the D19N/D40N/E43Q variant in 1 M KCl has a pKa of
5.0 (Table 2.1), which is 1.1 pH units higher than the pKa of Asp in water. Assuming
that high salt screens Coulomb interactions with other charges without affecting
dehydration energies or short-range interactions with other polar groups, the pKa of
Asp-21 in this variant at high salt provides evidence that its intrinsic pKa is also
elevated significantly. Therefore, it appears that Asp-21 behaves like the red group
in Figure 2.6 and that in the presence of repulsive Coulomb interactions its pKa is
shifted even further, whereas Asp-21 has little or no influence on the pKa values of
the other carboxylic groups. To better demonstrate this we measured titration
curves for Asp-19 and Asp-21 in variants with all other ionizable groups in the
active site neutralized (∆+PHS/R35Q/D40N/E43Q, ∆+PHS/D19N/R35Q/D40N/
E43Q, and ∆+PHS/D21N/R35Q/D40N/E43Q, Table 2.1 and Figure 2.7).
This
allowed us to examine in greater detail the interactions between two carboxylic
groups
within
the
context
of
a
protein.
The
pKa
of
Asp-19
in
∆+PHS/D21N/R35Q/D40N/E43Q approximates the intrinsic pKa of Asp-19 and has
a value of 3.46. This is lower than the normal pKa for Asp in water, and presumably
reflects the ability of Asp-19 to form a favorable hydrogen bond with the Asp-21 HN.
Similarly the pKa of Asp-21 in ∆+PHS/D19N/R35Q/D40N/E43Q has a pKa of 4.65,
which is higher than the pKa of Asp in water
60
182
180
Chemical Shift (ppm)
178
176
Asp-19
182
180
178
176
Asp-21
1
2
3
4
5
6
7
8
9
pH
Figure 2.7: Titration curves for Asp-19 (top) and Asp-21 (bottom) in
∆+PHS/D21N/R35Q/D40N/E43Q (red), ∆+PHS/D19N/R35Q/D40N/E43Q (blue),
and ∆+PHS/R35Q/D40N/E43Q (green). Lines represent fits of equations (2.2) or
(2.3) to the data. Equation (2.3) was used to fit Asp-19 in
∆+PHS/R35Q/D40N/E43Q; equation (2.2) was used to fit all other curves.
61
According to the thermodynamic cycle described above, a Coulomb
interaction between Asp-19 and Asp-21 should result in a large increase in the pKa
of Asp-21, because it has the higher intrinsic pKa, and a small, if any, increase in the
pKa of Asp-19. Consistent with this model, when both of these groups are present
(∆+PHS/R35Q/D40N/E43Q), the pKa of Asp-21 is 5.70, an increase of about one pH
unit relative to its pKa in the absence of Asp-19. On the other hand, Asp-19 has a pKa
of 3.10, which is 0.36 pH units lower than its pKa in the absence of Asp-21 (Figure
2.7). This difference cannot be explained in terms of the Coulomb interaction alone,
and suggests that the pKa of Asp-19 in the presence of Asn-21 is not exactly equal to
the “true” intrinsic pKa of Asp-19 (and perhaps likewise for the intrinsic pKa of Asp21). Because of this discrepancy we do not know how much of the measured pKa
shifts are due to Coulomb interactions versus other effects, and thus we cannot
determine the energy of the Coulomb interaction ∆GAB from the measured pKa shifts
alone. Nevertheless, the results are in qualitative agreement with the above model:
the group with the higher intrinsic pKa (Asp-21) exhibits a large response to the
Coulomb interaction with Asp-19, whereas Asp-19, having the lower intrinsic pKa,
does not appear to sense the Coulomb interaction at all.
2.4.3 Implications for structure-based pKa calculations
In FDPB calculations, the determinants of the pKa for a group are separated
into contributions from hydration, dipoles, and other ionizable groups according to
the following formalism:
62
(2.1)
where pKaW is the pKa of model compounds in water, ∆pKaBorn is the shift in pKa
caused by changes in hydration (Born energy), ∆pKabg is the background energy
arising from the interaction of the charge with the permanent dipoles of the protein,
and ∆pKa,ij is the contribution from the Coulomb interaction between groups i and j.
The self-energy, ∆pKa,ii is the sum of the Born and background terms, and the
intrinsic pKa, (pKaint) is the sum of the model compound pKa and the self-energy.
FDPB calculations applied to static structures exaggerate the magnitude of
Coulomb interactions between carboxylic groups and basic groups, and hence the
calculated pKa values of carboxylic groups tend to be more depressed than the
measured ones.8,17
What is not well appreciated is that when cooperative
interactions are present, if the intrinsic pKa values, which are typically calculated
first, are not calculated correctly, the resulting pKa values will not be correct even if
the magnitude of Coulomb interaction energies are calculated perfectly. To examine
the difficulties structure-based pKa calculations have in simultaneously calculating
self- and Coulomb energies, calculations were performed on ∆+PHS SNase using
different implementations of continuum electrostatics with the finite difference
Poisson-Boltzmann algorithm (FDPB) using static crystal structures and MD-relaxed
structures. In FDPB calculations using in = 20 and the default tautomeric state for
the cluster residues, the intrinsic pKa values are 4.50 for Asp-21, 3.52 for Asp-19,
63
4.06 for Asp-40 and 4.19 for Glu-43. This underestimates the intrinsic pKa of Asp-21
of 5.01 determined from the D19N/D40N/E43Q variant at 1 M KCl, conditions
under which Coulomb contributions to the pKa of Asp-21 were minimized. These
small errors in the calculations of intrinsic pKa values are sufficient to preclude
accurate estimation of the shifts in pKa values in the cluster.
In general, the best agreement between calculated and measured pKa values
are obtained when the protein is treated with in = 20.18,19 However, this approach
fails when applied to active sites and other ionizable clusters. In SNase, using in =
20 cannot reproduce the pKa values of the carboxylic groups in the cluster,
regardless of which crystal structure is used.8,17 In fact, for Asp-21, increasing the
value of the protein dielectric constant above 10 worsens agreement with
experiment (Figure 2.8).
The problem is that high values of in improve the
treatment of Coulomb interactions by attenuating them, but this improvement is
offset by the attenuation of the self-energy, which may reduce the accuracy of the
calculated intrinsic pKa values and hence offset any improvement in accuracy
resulting from attenuation of the Coulomb interactions. Note that the pKa values of
Asp-19 and Asp-21, which according to the calculations are under the influence of a
significant, destabilizing Born energy, are only reproduced with effective dielectric
constants less than 20, and Asp-21 only with a dielectric constant between 10 and
12 (Figure 2.8). Meanwhile, the pKa value of Asp-40 is insensitive to in > 8, and that
of Glu-43 is independent of the value of in.
The calculations show that the
particular value of the protein dielectric constant needed to reproduce the pKa of a
given ionizable group may depend on the extent to which the different terms in
64
Figure 2.8. (following page) FDPB pKa calculations shown as a function of in for the
four carboxylic groups in the active site cluster. Solid circles refer to pKa values
calculated with the structure of ∆+PHS (PDB accession code 3BDC) with the color
indicating the tautomeric state of the neutral form of the group: black, protonated
Oδ2 or Oε2 (state 1); blue, protonated Oδ1 or Oε1 (state 2); red, protonated Oδ1 for
Asp-19 only (state 3); green, protonated Oδ1 for Asp-21 only (state 4). Open circles
describe contributions to the calculated pKa shifts from Coulomb (black), self (blue),
Born (red), and background (green) interactions (using the protonated Oδ1/Oε1
states for all residues, which gives the best agreement with the experimentally
measured values at in = 10-12) The dotted black line is the model compound pKa,
and the solid line is the experimentally measured value from Table 2.1. The dashed
vertical lines denote the range of in values that reproduce the experimental pKa of
Asp-21 to within 0.5 pH units.
65
66
equation (2.1) contribute to its pKa. As others have suggested, different values of
the dielectric constant appear to be needed for the self-energy and for the Coulomb
interactions.20
The data in Figure 2.9 suggest that relaxing structures with classical MD
simulations is not always a reliable way to improve pKa calculations, at least not in
ionizable clusters or other cases where proton binding is highly cooperative.
Simulations in which the charged state of a single residue differs can lead to
significantly different conformations and to incorrect pKa values. Two 10 ns MD
simulations were performed, one with Asp-21 charged and one with Asp-21 neutral
(all other ionizable groups were assigned the charged states of their respective
model compounds at pH 7). pKa values were calculated at 50 ps intervals using in =
10. In the trajectory with Asp-21 charged (Figure 2.9(a)), the simulation settled into
a state with a very depressed pKa for Asp-21 (average value 0.9), mostly from
forming a strong apparent ion pair with Arg-35. On the other hand, in the trajectory
with neutral Asp-21 (Figure 2.9(b)) the simulation settled into a state with a high
pKa for Asp-21 (average value 5.9), coincident with a slight increase in both the Born
and background terms. Notably there was an abrupt decrease in the distance
between Asp-21 and the carbonyl oxygen of Val-39 upon shifting to the high pKa
state, consistent with the formation of a hydrogen bond like the one seen in the
NVIAGA crystal structures (Figure 2.1(b)).
Using longer MD runs would not
eliminate the problem, given that the conformation of the active site appears to
depend on the protonation state of Asp-21. Since classical MD simulations have
fixed protonation states, then a single MD run would be unlikely to sample both
67
Figure 2.9. pKa values calculated in structures sampled along MD trajectories
performed with Asp-21 in the charged (a) or neutral (b) states. The calculated pKa
values (black) and contributions to the shift in pKa from Coulomb (green), Born
(red) and background (blue) energies are shown. The solid horizontal lines
correspond to the same values calculated with the static crystal structure in the
default tautomeric state using εin = 10 (Figure 2.8).
68
conformations regardless of the simulation length.
In the case of clustered groups with similar intrinsic pKa values and strong
Coulomb interactions, small errors in the calculated intrinsic pKa values can be
amplified dramatically in the partitioning of the Coulomb interactions (Figure 2.6).
This is a serious problem because calculations of both the Born and background
terms that constitute the intrinsic pKa in equation (2.1) depend on the fine details of
the protein structure, especially at the low values of in required to weight these
terms appropriately. At εin = 4 the difference between calculations with different
tautomeric states was as large as 2 pH units for Asp-19 and Asp-21 (Figure 2.8).
Crystal structures can be biased by crystal packing interactions that are not present
in solution.21 For instance, in many crystal structures of SNase the side chain of Lys70 from an adjacent molecule inserts into the active site and comes within
hydrogen-bonding distance of Asp-19, Asp-21, and Glu-43. Similarly, the structure
can be subtly dependent on pH as we suspect is the case for the conformations of
Arg-35 and Asp-21. These potential problems with the conformation of the native
state as described by crystal structures can reduce the accuracy of intrinsic pKa
calculations. It is difficult to calculate pKa values in cooperative clusters of ionizable
groups with similar intrinsic pKa values precisely because the capacity to amplify
small signals is the hallmark of cooperativity; small and unavoidable inaccuracies in
the calculations will be amplified as well.
69
2.5 Conclusions
The elevated pKa of Asp-21 in SNase is not merely the result of repulsive
Coulomb interactions with the other carboxylic groups in the active-site cluster.
Even when all of the other residues in the cluster are neutralized, the intrinsic pKa of
Asp-21 is still higher than the normal pKa of Asp in water. Longer-range Coulomb
interactions cannot account for this effect, as screening of Coulomb interactions
with 1 M salt was not sufficient to reduce the pKa of Asp-21 to a normal value. The
intrinsic pKa of Asp-21 is elevated; consequently, Asp-21 senses repulsive
interactions with Asp-19, Asp-40, and Glu-43. Conversely, the pKa values of the
other carboxylic groups are not affected by the charge state of Asp-21 because they
all titrate in a pH range where Asp-21 is neutral. Asp-19 has a depressed pKa, due to
a combination of its acting as a hydrogen bond acceptor and a strong Coulomb
attraction to Arg-35. Asp-40 and Glu-43 have near-normal pKa values, which likely
reflect an equal balance of favorable and unfavorable interactions.
The reasons for the elevated intrinsic pKa of Asp-21 are not obvious from
SNase crystal structures. The experimental data suggest that the microenvironment
around Asp-21 in the crystal structure may differ from that in solution; the strong
Coulomb interaction between Asp-19 and Arg-35 suggested by the increased pKa of
Asp-19 in R35Q is inconsistent with the relative positions of these groups in the
crystal structure. Crystal structures of variants with an Asn residue replacing Asp21 show Arg-35 in an altered conformation that places it much closer to Asp-19.
These same variants have Asn-21 donating a hydrogen bond to a backbone
carbonyl. If Asp-21 were similarly capable of donating a hydrogen bond in the
70
protonated state, then such an interaction would favor the protonated state of Asp21 and lead to an upward shift in its intrinsic pKa.
The ability of hydrogen bonds to depress the pKa values of Asp and Glu
residues acting as hydrogen bond acceptors is well documented.15,21 The effect on
the pKa when a protonated Asp or Glu acts as a hydrogen bond donor has not been
well established, but it seems reasonable to expect that such an interaction would
favor the protonated state and elevate the pKa. Since protein hydrogen atoms are
not generally visible by x-ray crystallography, assumptions must be made about the
protonation states of Asp and Glu residues when performing calculations on x-ray
structures, which may not necessarily reflect the actual protonation states of those
residues.
Therefore it will be very difficult for calculations based on crystal
structures to predict the consequences of hydrogen bonds formed by these residues
without some means of determining the correct protonation states. An algorithm
for placing hydrogens in crystal structures based on global optimization of hydrogen
bond network for each protonation state has been shown to improve the accuracy of
pKa calculations for active site residues of Bacillus circulans xylanase and hen egg
white lysozyme.21,22
The active site cluster of SNase may provide a useful
benchmark for further calibrating such a method.
2.6 Materials and methods
71
2.6.1 Protein expression and purification
All experiments were performed with a highly stable, acid-resistant variant
of SNase known as ∆+PHS to ensure that the protein remained folded throughout
the titration of most carboxylic groups.8 Site-directed mutagenesis was performed
using the QuickChange kit (Stratagene).
uniformly
13C/15N
For NMR titrations, all variants were
labeled and expressed as described previously.8 All proteins
were purified according to the procedure of Shortle and Meeker.23
2.6.2 NMR spectroscopy
For pH titrations, protein samples were prepared by exchanging from H2O
into aqueous solution containing 100 mM KCl (or 1 M KCl), 10% D2O, and 0.5 mM
NaN3. Exchange was conducted by successive dilutions in Amicon Ultra-4 tubes
(Millipore). Final protein concentrations ranged from 0.8 to 1.1 mM, as measured
by absorbance at 280 nm using an extinction coefficient of 0.93 (mg/ml)-1. A 1.4 mL
volume of sample was prepared and divided into two equal fractions, one to be
initially titrated with acid and the other with base. Titrations were carried out as
described previously.8 The chemical shifts of the carboxyl carbons of Asp and Glu
side chains, as well as those of the adjacent methylene carbons, were monitored
using a two-dimensional
13C-detect
CBCGCO experiment.8
assignments, as well as Asp and Glu side chain
were determined previously.8
13C
Full backbone
assignments in ∆+PHS SNase
The resonances in the CBCGCO spectra for the
variants could be assigned by comparison with the spectrum for ∆+PHS.
72
Side chain proton assignments were obtained using standard tripleresonance HBHA(CO)NH and H(CCCO)NH-TOCSY experiments. The sample buffer
consisted of 25 mM potassium acetate, 100 mM KCl, 10% D2O, and 0.5 mM NaN3.
The final protein concentration was 0.7 mM, and the final sample pH was 4.68. The
same sample was used to collect a 3D 15N-NOESY-HSQC with a mixing time of 75 ms.
Titrations, HBHA(CO)NH, and H(CCCO)NH-TOCSY experiments were collected on a
Bruker Avance II 600 equipped with a cryogenic-TCI probe (with a cryocooled
13C
preamplifier), whereas the NOESY was collected on a Bruker Avance 600 equipped
with a cryogenic-TXI probe. All experiments were collected at 298 K and in the
absence of Ca2+ or pdTp.
2.6.3 pKa values
pKa values were obtained from the pH dependence of the C (Asp) or C (Glu)
chemical shifts by fitting a modified Hill equation24 to the data:
obspH
AH A 10 n pHpK
a
(2.2)
110 npHpK a
AH and A- are the chemical shifts of the protonated and deprotonated forms of the
residue, respectively. The parameter n is the Hill coefficient that describes the slope
of the titration curve in the transition region, and reflects the degree of
cooperativity in binding.
This analysis assumes that the protonated and
deprotonated states are in fast exchange. Non-linear least squares fitting was
73
performed with the nlme library in the R statistics package.25 In cases where two
titration events were evident in the curve, a two-site modified Hill equation was
used:26
obspH
AH 2 AH 10n1pHpK A 10n1pHpK
a1
a1
n 2pHpK a 2
110 n1pHpK a1 10 n1pHpK a1 n 2pHpK a 2
(2.3)
This model differs from the two-site model used by Castañeda et al., which did not
include Hill coefficients.8 The two pKa values and Hill coefficients correspond to the
separate titration events. To minimize the number of fitting parameters, the Hill
coefficient of the smaller titration event was fixed to be 1, since in all cases the
amplitude of the smaller titration was too small for the Hill coefficient to be fit
accurately.
pH values were not corrected for deuterium isotope effects.
For
residues for which complete titration curves could not be obtained because of
protein unfolding at low pH, the amplitude A AH of the titration was fixed
to the value determined for the same residue at 1 M KCl, as described previously. 8
In the case of Glu-75, whose resonance broadened beyond detection below pH 4 at 1
M KCl, the pKa was obtained by fixing the amplitude of the titration to that obtained
at 100 mM KCl. For Asp-77 and Asp-83 pKa values could not be obtained because
these groups titrate at a pH value below the acid-unfolding midpoint of the protein
in all variants.8 However, for the Asp-77 pKa in most variants an upper bound was
74
assigned using by fitting equation (2.3) with the Hill coefficients fixed to 1 and ∆
fixed to the smallest value observed for other Asp residues.
For the reference protein, ∆+PHS, we applied the fitting procedure described
above to the data from Castañeda et al.8 These data at 100 mM KCl comprise three
separate titration experiments, and the mean and standard errors of pKa values and
Hill coefficients over these three experiments are reported in Table 2.1 and
Appendix A. Small discrepancies between the results from Castañeda et al. and
those reported here result from using a different model for two-site fits, as
explained above, and using the two-site model for residues other than Asp-19 and
Asp-21.
The largest standard error observed was 0.06 pH units, which is of
comparable magnitude to the errors of each individual fit. Therefore, for the other
variants and conditions, a single titration experiment was performed and the pKa
values and Hill coefficients with fitting errors from this experiment are reported.
2.6.4 Comparison of SNase structures
Crystal structures of 119 SNase variants from the PDB were analyzed (1A2T,
1A2U, 1A3T, 1A3U, 1A3V, 1AEX, 1ENA, 1ENC, 1EQV, 1EY0, 1EY4, 1EY5, 1EY6, 1EY7,
1EY8, 1EY9, 1EYA, 1EYC, 1EYD, 1EZ6, 1EZ8, 1F2M, 1F2Y, 1F2Z, 1IHZ, 1II3, 1KAA,
1KAB, 1KDA, 1KDB, 1KDC, 1NSN, 1NUC, 1SNC, 1SND, 1SNM, 1SNO, 1SNP, 1SNQ,
1STA, 1STB, 1STG, 1STH, 1STN, 1STY, 1SYB, 1SYC, 1SYD, 1SYE, 1SYF, 1SYG, 1TQO,
1TR5, 1TT2, 1U9R, 2ENB, 2EXZ, 2EY1, 2EY2, 2EY5, 2EY6, 2EYF, 2EYH, 2EYJ, 2EYL,
2EYM, 2EYO, 2EYP, 2F0D, 2F0E, 2F0F, 2F0G, 2F0H, 2F0I, 2F0J, 2F0K, 2F0L, 2F0M,
2F0N, 2F0O, 2F0P, 2F0Q, 2F0S, 2F0T, 2F0U, 2F0V, 2F0W, 2NUC, 2OEO, 2OF1, 2OXP,
75
2PW5, 2PW7, 2PYK, 2PZT, 2PZU, 2PZW, 2QDB, 2RBM, 2RDF, 2RKS, 2SNM, 2SNS,
3BDC, 3C1E, 3C1F, 3D4D, 3D4W, 3D6C, 3D8G, 3DHQ, 3DMU, 3E5S, 3EJI, 3ERO, 3ERQ,
3EVQ, 3NUC, 5NUC). All structures have resolutions between 1.5 and 2.8 Å. The
distance from Arg-35 to Asp-19 and Asp-21 were measured in each structure using
as endpoints the Arg-35 C and the midpoint between the O atoms of Asp-19 or
Asp-21.
Histograms of the resulting values for both Asp-19 and Asp-21 were
generated using R.25
2.6.5 Crystal structure of ∆+PHS/D21N
Crystals were grown at 277 K using hanging drop vapor diffusion methods.
The reservoir solution contained 38% (v/v) 2-methyl-2,4-pentanediol (MPD)
(Sigma-Aldrich) and 25 mM potassium phosphate buffer, pH 6.0. Protein of 23
mg/mL concentration was combined with CaCl2 and thymine 3’,5’-diphosphate
(pdTp) in a 1:3:2 molar ratio prior to mixing equal volumes with the reservoir
solution and equilibrating at 4 °C. Crystals were mounted in nylon loops on a
copper base (CryoloopsTM and CrystalCap Copper MagneticTM, Hampton Research),
flash-cooled in liquid nitrogen and stored at 78 K.
Diffraction data were collected from a single crystal using beamline X-25 at
the National Synchrotron Light Source. Data were indexed, integrated, and scaled
using HKL2000.27 Initial phases were obtained by molecular replacement using the
program Phaser28 within the CCP4 suite.29
The coordinates for ∆+PHS (PDB
accession code 3BDC) with heteroatoms removed, B-factors set to 20 Å2, and
residue 21 truncated to Ala. Several alternating rounds of structure refinement
76
using Refmac530 and model building using Coot31 resulted in a final model that
contained residues 1-142, one pdTp molecule, one phosphate ion, and 143 water
molecules. Only water molecules for which both 2Fo-Fc and Fo-Fc electron density
(contoured to 1.2 s and 3.0 s, respectively) were observed and that were within 3.5
Å of a likely hydrogen-bonding partner were incorporated into the model. Data
collection and refinement statistics are summarized in Appendix A.
2.6.6 Structure-based continuum electrostatic calculations
Calculations were performed with the structure of ∆+PHS SNase (3BDC).8
pKa values were calculated using the FDPB method with the linearized form of the
Poisson-Boltzmann equation, as implemented in the University of Houston
Brownian Dynamics package of McCammon and co-workers.18,19,32,33 Details of
FDPB calculations with SNase have been presented elsewhere.17,34 Energies were
computed using the distributed charge scheme for the ionizable form of each
titratable residue.19,35–38 Calculations with both the PARSE parameter set39 and the
CHARMm version 22 polar hydrogen-only parameter set40,41 were compared (data
not shown); the reported results are those for the PARSE parameter set. The default
placement of hydrogen atoms was on O2 for all Asp residues and O2 for all Glu
residues, unless noted otherwise.
FDPB pKa calculations were also computed on 50 ps snapshots of 10 ns MD
trajectories. The trajectories were computed using the program NAMD242 and the
CHARMm v27 forcefield.41 The system was simulated with explicit solvent (TIP3
77
water) and periodic boundary conditions, using the particle mesh Ewald method
with real space interaction cutoff of 10 Å.
78
2.7 References
1. Søndergaard, C.R., McIntosh, L.P., Pollastri, G. & Nielsen, J.E. (2008).
Determination of electrostatic interaction energies and protonation state
populations in enzyme active sites. Journal of Molecular Biology 376, 269–287
2. Ondrechen, M.J., Clifton, J.G. & Ringe, D. (2001). THEMATICS: A simple
computational predictor of enzyme function from structure. Proceedings of the
National Academy of Sciences of the United States of America 98, 12473–12478
3. McIntosh, L.P., Hand, G., Johnson, P.E., Joshi, M.D., Körner, M., Plesniak, L.A., et al.
(1996). The pKa of the general acid/base carboxyl group of a glycosidase cycles
during catalysis: a 13C-NMR study of bacillus circulans xylanase. Biochemistry
35, 9958–9966
4. Ackers, G.K., Shea, M.A. & Smith, F.R. (1983). Free energy coupling within
macromolecules: The chemical work of ligand binding at the individual sites in
co-operative systems. Journal of Molecular Biology 170, 223–242
5. Weber, D.J., Gittis, A.G., Mullen, G.P., Abeygunawardana, C., Lattman, E.E. &
Mildvan, A.S. (1992). NMR docking of a substrate into the X-ray structure of
staphylococcal nuclease. Proteins: Structure, Function, and Genetics 13, 275–287
6. Serpersu, E.H., Shortle, D. & Mildvan, A.S. (1987). Kinetic and magnetic
resonance studies of active-site mutants of staphylococcal nuclease: factors
contributing to catalysis. Biochemistry 26, 1289–1300
7. Serpersu, E.H., Hibler, D.W., Gerlt, J.A. & Mildvan, A.S. (1989). Kinetic and
magnetic resonance studies of the glutamate-43 to serine mutant of
staphylococcal nuclease. Biochemistry 28, 1539–1548
8. Castañeda, C.A., Fitch, C.A., Majumdar, A., Khangulov, V., Schlessman, J.L. &
García‐Moreno, B.E. (2009). Molecular determinants of the pKa values of Asp
and Glu residues in staphylococcal nuclease. Proteins: Structure, Function, and
Bioinformatics 77, 570–588
9. Baran, K.L., Chimenti, M.S., Schlessman, J.L., Fitch, C.A., Herbst, K.J. & GarcíaMoreno, B. (2008). Electrostatic effects in a network of polar and ionizable
groups in staphylococcal nuclease. Journal of Molecular Biology 379, 1045–1062
10. Rule, G.S. & Hitchens, T.K. (2006). Fundamentals of Protein NMR Spectroscopy.
Springer, Dordrecht.
11. Chen, J., Lu, Z., Sakon, J. & Stites, W.E. (2000). Increasing the thermostability of
staphylococcal nuclease: implications for the origin of protein thermostability.
Journal of Molecular Biology 303, 125–130
12. Berman, H.M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T.N., Weissig, H., et al.
(2000). The Protein Data Bank. Nucleic Acids Research 28, 235–242
13. Chen, V.B., Arendall, W.B., Headd, J.J., Keedy, D.A., Immormino, R.M., Kapral, G.J.,
et al. (2009). MolProbity: all-atom structure validation for macromolecular
crystallography. Acta Crystallographica Section D-Biological Crystallography 66,
12–21
14. Davis, I.W., Leaver-Fay, A., Chen, V.B., Block, J.N., Kapral, G.J., Wang, X., et al.
(2007). MolProbity: all-atom contacts and structure validation for proteins and
nucleic acids. Nucleic Acids Research 35 Suppl 2, W375–W383
79
15. Forsyth, W.R., Antosiewicz, J.M. & Robertson, A.D. (2002). Empirical
relationships between protein structure and carboxyl pKa values in proteins.
Proteins: Structure, Function, and Genetics 48, 388–403
16. Bombarda, E. & Ullmann, G.M. (2010). pH-dependent pKa values in proteins—a
theoretical analysis of protonation energies with practical consequences for
enzymatic reactions. The Journal of Physical Chemistry B 114, 1994–2003
17. Fitch, C.A., Whitten, S.T., Hilser, V.J. & García‐Moreno E., B. (2006). Molecular
mechanisms of pH‐driven conformational transitions of proteins: Insights from
continuum electrostatics calculations of acid unfolding. Proteins: Structure,
Function, and Bioinformatics 63, 113–126
18. Antosiewicz, J., McCammon, J.A. & Gilson, M.K. (1994). Prediction of Phdependent Properties of Proteins. Journal of Molecular Biology 238, 415–436
19. Antosiewicz, J., McCammon, J.A. & Gilson, M.K. (1996). The determinants of pKas
in proteins. Biochemistry 35, 7819–7833
20. Schutz, C.N. & Warshel, A. (2001). What are the dielectric “constants” of proteins
and how to validate electrostatic models? Proteins: Structure, Function, and
Bioinformatics 44, 400–417
21. Nielsen, J.E. & Vriend, G. (2001). Optimizing the hydrogen-bond network in
Poisson-Boltzmann equation-based pKa calculations. Proteins: Structure,
Function, and Genetics 43, 403–412
22. Nielsen, J.E., Andersen, K.V., Honig, B., Hooft, R.W.W., Klebe, G., Vriend, G., et al.
(1999). Improving macromolecular electrostatics calculations. Protein
Engineering 12, 657–662
23. Shortle, D. & Meeker, A.K. (1986). Mutant forms of staphylococcal nuclease with
altered patterns of guanidine hydrochloride and urea denaturation. Proteins:
Structure, Function, and Genetics 1, 81–89
24. Markley, J.L. (1975). Observation of histidine residues in proteins by nuclear
magnetic resonance spectroscopy. Accounts of Chemical Research 8, 70–80
25. R Development Core Team (2009). R: A language and environment for statistical
computing. R Foundation for Statistical Computing, Vienna, Austria. at
<http://www.R-project.org>
26. Pérez-Cañadillas, J.M., Campos-Olivas, R., Lacadena, J., Martínez del Pozo, A.,
Gavilanes, J.G., Santoro, J., et al. (1998). Characterization of pKa values and
titration shifts in the cytotoxic ribonuclease alpha-sarcin by NMR. Relationship
between electrostatic interactions, structure, and catalytic function.
Biochemistry 37, 15865–15876
27. Otwinowski, Z. & Minor, W. (1997). Processing of X-ray diffraction data collected
in oscillation mode. Methods in Enzymology 276, 307–326
28. McCoy, A.J., Grosse-Kunstleve, R.W., Storoni, L.C. & Read, R.J. (2005). Likelihoodenhanced fast translation functions. Acta Crystallographica Section D-Biological
Crystallography 61, 458–464
29. Bailey, S. (1994). The CCP4 suite: programs for protein crystallography. Acta
Crystallographica Section D-Biological Crystallography 50, 760–763
30. Murshudov, G.N., Vagin, A.A. & Dodson, E.J. (1997). Refinement of
macromolecular structures by the maximum-likelihood method. Acta
Crystallographica Section D-Biological Crystallography 53, 240–255
80
31. Emsley, P. & Cowtan, K. (2004). Coot: model-building tools for molecular
graphics. Acta Crystallographica Section D-Biological Crystallography 60, 2126–
2132
32. Davis, M.E., Madura, J.D., Luty, B.A. & McCammon, J.A. (1991). Electrostatics and
diffusion of molecules in solution: simulations with the University of Houston
Brownian dynamics program. Computer Physics Communications 62, 187–197
33. Madura, J.D., Briggs, J.M., Wade, R.C., Davis, M.E., Luty, B.A., Ilin, A., et al. (1995).
Electrostatics and diffusion of molecules in solution: simulations with the
University of Houston Brownian Dynamics program. Computer Physics
Communications 91, 57–95
34. Fitch, C.A., Karp, D.A., Lee, K.K., Stites, W.E., Lattman, E.E. & García-Moreno, E.B.
(2002). Experimental pKa Values of Buried Residues: Analysis with Continuum
Methods and Role of Water Penetration. Biophysical Journal 82, 3289–3304
35. Bashford, D. & Gerwert, K. (1992). Electrostatic calculations of the pKa values of
ionizable groups in bacteriorhodopsin. Journal of Molecular Biology 224, 473–
486
36. Yang, A.-S. & Honig, B. (1993). On the pH dependence of protein stability. Journal
of Molecular Biology 231, 459–474
37. Antosiewicz, J., Briggs, J.M., Elcock, A.H., Gilson, M.K. & McCammon, J.A. (1996).
Computing ionization states of proteins with a detailed charge model. Journal of
Computational Chemistry 17, 1633–1644
38. Trylska, J., Antosiewicz, J., Geller, M., Hodge, C.N., Klabe, R.M., Head, M.S., et al.
(1999). Thermodynamic linkage between the binding of protons and inhibitors
to HIV-1 protease. Protein Science 8, 180–195
39. Sitkoff, D., Sharp, K.A. & Honig, B. (1994). Accurate calculation of hydration free
energies using macroscopic solvent models. The Journal of Physical Chemistry
98, 1978–1988
40. Brooks, B.R., Bruccoleri, R.E., Olafson, B.D., States, D.J., Swaminathan, S. &
Karplus, M. (1983). CHARMM: A program for macromolecular energy,
minimization, and dynamics calculations. Journal of Computational Chemistry 4,
187–217
41. MacKerell, A.D., Bashford, D., Bellott, M., Dunbrack, R.L., Evanseck, J.D., Field, M.J.,
et al. (1998). All-atom empirical potential for molecular modeling and dynamics
studies of proteins. The Journal of Physical Chemistry B 102, 3586–3616
42. Kalé, L., Skeel, R., Bhandarkar, M., Brunner, R., Gursoy, A., Krawetz, N., et al.
(1999). NAMD2: Greater scalability for parallel molecular dynamics. Journal of
Computational Physics 151, 283–312
81
3 Conformational Reorganization of
the Backbone Influences the pKa
Values of Ionizable Groups in
Proteins
(to be submitted in a slightly different form to Journal of the American Chemical
Society under the authorship of Brian M. Doctrow, Jamie L. Schlessman, Ananya
Majumdar, and Bertrand García-Moreno E.)
82
3.1 Abstract
The pKa values of ionizable residues at protein surfaces are usually similar to
those of ionizable residues in water. However, electrostatics calculations with static
structures tend to predict large shifts in pKa values.
These problems can be
minimized by using artificially high dielectric constants, but sometimes the
discrepancy persists even when the protein interior is treated with the dielectric
constant of water. This suggests that the conformational dynamics of the protein,
which are not reflected in the crystal structure, are reflected in pKa values.
Molecular dynamics or Monte Carlo simulations can attempt to reproduce these
dynamic effects, but there are no useful data for testing this approach. To examine
the role of backbone conformational heterogeneity and reorganization in
determining pKa values, NMR spectroscopy was used to measure the pKa values of
all 20 Asp and Glu residues in variants of staphylococcal nuclease (SNase) with Gly
substitutions at select locations. These substitutions were intended to enhance
backbone reorganization without affecting the overall conformation of the protein.
Some of the Gly substitutions tested shifted the pKa values of nearby carboxylic
groups without having a significant effect on the crystal structure.
Hydrogen
exchange measurements indicated increased propensity for local unfolding near the
residues whose pKa values were affected by the Gly substitutions. Calculations with
continuum electrostatic methods using crystal structures of the variants do not
reproduce the effects of Gly substitutions on pKa values. Our results suggest that the
high apparent polarizability of proteins might be the result of subtle structural
reorganization of the backbone that is difficult to reproduce computationally.
83
3.2 Introduction
Ionizable groups in proteins play essential roles in processes such as
catalysis1, H+ transport2–4, and the regulation of function by pH5,6. To understand
how ionizable groups contribute to biochemical processes, it is necessary to know
their pKa values and to understand the factors that govern them. To identify the
molecular determinants of the pKa values we usually depend on structure-based
pKa calculations that attempt to reproduce pKa values solely from protein structure
and based on physical principles7–14. The success of these methods at reproducing
experimental pKa values has been limited15, suggesting that the physical
determinants of pKa values are still not fully understood. Here we examine how the
conformational heterogeneity of the backbone and its high capacity for
reorganization affect pKa values. This is something that is very difficult to treat
computationally and which has not been examined systematically before.
The pKa values of surface ionizable groups tend to be similar to the normal
values of model compounds in water16–18. This is difficult to reproduce using
calculations with static structures, which tend to predict pKa values significantly
shifted from their model compound values19. The discrepancy can be minimized by
using an arbitrarily high protein dielectric constant, presumably because the high
dielectric constant implicitly accounts for protein reorganzation11. However, in
many cases the calculated pKa values do not match the measured values even when
the protein is treated with the dielectric constant of water15,18. This implies that the
relative positions of ionizable groups in the crystal structure do not match the
average positions of the ensemble in solution. The conformational properties of the
84
ensemble in solution must be treated explicitly in calculations to improve their
accuracy.
Structure-based calculations can treat conformational reorganization
explicitly with molecular dynamics (MD) simulations20 or Monte Carlo (MC)
methods12,21. Although these methods can improve the accuracy of pKa calculations,
often this requires arbitrary adjustments to the dielectric constant to achieve
acceptable accuracy. In some cases the calculations fail. Neither MD nor MC
methods sample backbone conformational changes adequately: the former because
the timescale of reorganziation of the backbone can be long, and the latter because
the conformational space is too large. If the inherent heterogeneity of the backbone
and its ability to reorganize can affect pKa values, then neither MD nor MC are likely
to be an effective approach to the improvement of structure-based calculations.
Previously, an ensemble model based on the COREX algorithm22 was able to
reproduce the acid unfolding profile of staphylococcal nuclease (SNase) better than
electrostatic calculations with a static structure. It was also able to identify the
carboxylic residues responsible for acid unfolding23. In this model the protein is
treated as a Boltzmann-weighted ensemble of partially unfolded structures, and
ionizable groups are assigned different pKa values depending on whether they are in
folded or unfolded regions of the protein. The overall pKa of a residue then reflects
an average over all of the states:
pKa P1 pK1a P 2 pKa2 K
(1)
85
where Pi is the population of state i, and pKai the pKa of the residue in state i. In this
model, the more likely a residue is to be unfolded, the more normal its pKa will be.
The model essentially captures the contributions of local unfolding and backbone
reorganziation on pKa values. The success of this model in reproducing the acid
unfolding of SNase suggests that the conformational heterogeneity and stability of a
carboxylic group’s microenvironment can significantly influence its pKa.
This
remains to be demonstrated experimentally.
To examine how conformational heterogeneity and reorganization of the
protein backbone can affect pKa values of ionizable groups, we measured the pKa
values of Asp and Glu residues in SNase in variants with Gly substitutions. Glycine is
known to promote fluctuations and local unfolding of the protein backbone24,25. Our
results show that glycine substitutions can affect on pKa values significantly, without
altering the charges or polarity near the affected ionizable groups and without
detectable changes to the protein conformation in the crystal structure.
pKa
calculations that represent the protein with a single structure are unable to
reproduce the shifts in pKa values caused by the substitutions with Gly. Hydrogendeuterium exchange suggests that the pKa shifts are associated with increased
backbone fluctuations, consistent with the view that conformational reorganziation
can modulate the electrostatic microenvironment of an ionizable group and act as
an important determinant of pKa values.
3.3 Results
86
Figure 3.1 shows the locations of the different Gly substitution sites and of
Asp and Glu side chains. The initial hypothesis was that increased fluctuations
promoted by Gly substitutions would allow ionizable groups to sample more
solvent-exposed environments, therefore the substitutions should tend to normalize
pKa values. The glycine substitutions were thus chosen to target residues whose pKa
is significantly depressed in ∆+PHS18. Only hydrophobic residues were substituted
to ensure that any observed pKa shifts were not caused by direct removal of polar or
Coulomb interactions. Substitutions of residues within the hydrophobic core were
largely avoided so as not to disrupt the overall folding of the protein.
3.3.1 pKa values measured by NMR spectroscopy
The pKa values of Asp and Glu residues were determined from the pHdependence of the carboxyl carbon chemical shifts measured with a CBCGCO
experiment. The resulting titration curves are shown in Figure 3.2(a) for a subset of
the Asp and Glu residues. The corresponding pKa values are listed in Table 3.1.
Using the stable form of SNase known as ∆+PHS as the reference state ensured that
the protein remained folded over as wide a pH range as possible. Nevertheless,
there were several instances where the low pH-baseline for a titration curve could
not be determined because the protein unfolded before the residue was fully
protonated.
In such cases, we followed the procedure of Castañeda et al. by
assuming that the amplitude of the titration did not vary with salt concentration and
fixing the lower baseline to match the amplitudes measured at 1M KCl18.
87
Figure 3.1: Cα trace of ∆+PHS SNase (PDB accession code 3BDC). Asp and Glu side
chains are shown as ball-and-stick representation. Colored spheres indicate
locations of Gly substitutions: Pro-11 (white), Ala-60 (blue), Ala-69 (red), Met-98
(green), and Ala-130 (gray). The color scheme will be maintained in subsequent
figures.
88
Figure 3.2: (a) Plots of carboxyl carbon chemical shift vs. pH for the subset of
carboxylic residues indicated in Figure 3.1 in each Gly-substituted variant. Lines
represent fits of the data to a two- or three-state modified Hill equation, as
described in the text. Dashed lines indicate that the fit was performed using a fixed
value for the lower baseline, as described in Castañeda et al.18 (b) Bar graphs
indicating the shift in pKa relative to the pKa measured in ∆+PHS. For Asp-77, the
difference in the upper limits for the pKa between the variant and ∆+PHS are shown.
89
Table 3.1. pKa values of select Asp and Glu residues measured by NMR
spectroscopy.a
Protein
Residue
pKab
∆pKac
d
e
∆+PHS
Asp-77
≤ 1.7
f
Asp-95
2.16 ± 0.04
Glu-57
3.49 ± 0.05
g
Glu-75
3.30 ± 0.02
Glu-101
3.81 ± 0.06
e
∆+PHS/P11G
Asp-77
≤ 1.6
Asp-95
2.15 ± 0.01f
-0.01 ± 0.04
Glu-57
3.45 ± 0.01
-0.04 ± 0.05
Glu-75
3.17 ± 0.05g
-0.13 ± 0.05
Glu-101
3.84 ± 0.01
0.03 ± 0.06
e
∆+PHS/A60G
Asp-77
≤ 1.7
Asp-95
2.38 ± 0.01f
0.22 ± 0.04
Glu-57
3.67 ± 0.01
0.18 ± 0.05
Glu-75
3.45 ± 0.01f,g
0.15 ± 0.02
Glu-101
3.98 ± 0.01
0.17 ± 0.06
∆+PHS/A58G/A60G
Asp-77
≤ 1.8e
f
Asp-95
1.93 ± 0.05
-0.23 ±0.06
Glu-57
3.61 ± 0.01f
0.12 ± 0.05
f,g
Glu-75
3.19 ± 0.01
-0.11 ± 0.02
Glu-101
3.83 ± 0.05
0.02 ± 0.08
e
∆+PHS/A69G
Asp-77
≤ 1.6
Asp-95
2.77 ± 0.02f
0.61 ± 0.04
Glu-57
3.52 ± 0.02
0.03 ± 0.05
Glu-75
3.27 ± 0.04g
-0.03 ± 0.04
Glu-101
3.77 ± 0.04
-0.04 ± 0.07
∆+PHS/M98G
Asp-77
≤ 2.5e
f
Asp-95
2.25 ± 0.05
0.09 ± 0.06
Glu-57
3.46 ± 0.07
-0.03 ± 0.09
Glu-75
3.92 ± 0.05
0.62 ± 0.05
f
Glu-101
3.32 ± 0.01
-0.49 ± 0.06
e
∆+PHS/M98A
Asp-77
≤ 2.7
Asp-95
2.13 ± 0.06f
-0.03 ± 0.07
Glu-57
3.43 ± 0.05
-0.06 ± 0.07
f
Glu-75
3.91 ± 0.16
0.60 ± 0.16
Glu-101
3.27 ± 0.02f
-0.54 ± 0.06
∆+PHS/A130G
Asp-77
≤ 1.6e
Asp-95
2.37 ± 0.01f
0.21 ± 0.04
Glu-57
3.61 ± 0.01
0.12 ± 0.05
Glu-75
3.50 ± 0.03g
0.20 ± 0.04
Glu-101
4.02 ± 0.01
0.21 ± 0.06
∆+PHS/A128G/A130G
Asp-77
≤ 1.8e
f
Asp-95
2.13 ± 0.04
-0.03 ± 0.06
Glu-57
3.67 ± 0.02
0.18 ± 0.05
90
Glu-75
Glu-101
3.31 ± 0.02f,g
3.97 ± 0.02h
0.00 ± 0.03
0.16 ± 0.06
Measurements were performed at 298 K and 100 mM KCl.
pKa values were obtained by fitting a single-site modified Hill equation to the data, unless otherwise
indicated. Values reported are from a single titration experiment with corresponding goodness of
fit, unless otherwise indicated.
c Change in pKa relative to ∆+PHS
d pKa values for ∆+PHS are means & standard errors over 3 independent titration experiments, using
the data from Castañeda et al18.
e Upper limit for Asp-77 pKa obtained by fitting the data to a two-site modified Hill equation with a
fixed Hill coefficient of 1 and a fixed ∆δ of 1.85 ppm
f Fit performed by fixing the amplitude of the titration (∆δ) to the value obtained from the titration of
the same residue in ∆+PHS at 1M KCl18 or at 100 mM (for Glu-75)
g pKa values obtained by fitting a two-site modified Hill equation to the data. Only values
corresponding to the larger of the two transitions are reported.
h Fit performed by fixing ∆δ to the largest value obtained for titration of other Glu residues (4.45
ppm)
a
b
91
The effects of Gly substitutions on the pKa values of Glu-57 are representative
of most of the Asp and Glu residues in SNase; the pKa is shifted from the ∆+PHS
value by no more than 0.25 pH units (corresponding to a change in protonation free
energy less that 0.34 kcal/mol). This is the case for all of the Asp & Glu residues not
shown in Figure 3.2 (Table B.1). The fact that the majority of pKa values in each Glycontaining variant are minimally perturbed suggests that the glycine substitutions
are not causing any global structural perturbations. As can be seen from Figure
3.2(b), only such minimal shifts were observed in three of the five variants (P11G,
A60G, A130G).
In two variants (A69G and M98G), the pKa of at least one residue was shifted
by 0.5 pH units or more relative to the reference protein. The pKa of Asp-95
increases by 0.6 pH units in A69G, while that of Glu-75 increases by the same
amount in M98G (Table 3.1 and Figure 3.2(b)). Both of these residues have pKa
values that are depressed in ∆+PHS relative to their model compound values (3.9 for
Asp, 4.4 for Glu). Thus, a positive ∆pKa corresponds to a shift towards a more
normal pKa value, consistent with the idea that the Gly substitutions are promoting
states in which these residues are more solvent exposed and not forming hydrogen
bonds or Coulomb interactions with the rest of the protein. In contrast, the pKa of
Glu-101 in M98G shifts away from the model compound pKa by 0.5 pH units,
suggesting that the Gly substitution allows this residue to form favorable
interactions that are not accessible to it in ∆+PHS.
The case of Asp-77 merits special mention. This residue’s pKa is below the
pH at which the protein unfolds; its pKa cannot be measured even in ∆+PHS.
92
However, enough of the beginning of the transition is observable to allow a
reasonable estimate of an upper limit for the pKa. The difference in these estimated
upper limits are what is plotted in Figure 3.2(b). The upper limit estimated in M98G
is 0.8 pH units higher than that in ∆+PHS, suggesting that Asp-77 begins to titrate at
a significantly higher pH in M98G than in ∆+PHS (Table 3.1). This in turn suggests
that the pKa of Asp-77 is higher in M98G than in ∆+PHS, even though a quantitative
measurement of pKa values is not possible for this residue.
The M98G substitution involves the removal of a bulky methionine side
chain. It is therefore possible that this substitution causes significant changes to
side chain packing and that this may be the cause of the increases in pKa rather than
an increase in backbone fluctuations.
To establish this, pKa values were also
measured in the M98A variant. The M98A substitution should have a similar effect
on side chain packing as M98G, but should not increase backbone fluctuations
appreciably since the substitution retains the Cβ. The same large shifts in pKa
measured in M98G were also observed in M98A (Figure B.1 and Table B.1). Thus
the large pKa shifts in the M98G variant appear to be a response to changes in side
chain packing interactions resulting from the removal of the Met-98 side chain.
Residues Ala-60 and Ala-130, where Gly substitution did not produce any pKa
shifts, are both located in the middle of helices. One might thus expect the backbone
at these positions to be relatively rigid regardless of the amino acid type, and thus a
single Gly substitution might not be enough to significantly increase backbone
fluctuations. Therefore, a second Gly substitution was introduced to each of the
A60G and A130G variants near the original substitution site to see if further
93
perturbations could produce a measureable response. Neither of the double-glycine
variants showed significant changes in pKa values (Figure B.1 and Table B.1). No
pKa shifts larger than 0.25 pH units were observed in the A58G/A60G variant
compared to ∆+PHS. In the A128G/A130G variant, the pKa of one residue (Glu-135)
shifted towards the model compound value by 0.33 pH units – larger than the shifts
observed in the corresponding single variant (A130G), but still much smaller than
the shifts observed in either A69G or M98G.
Therefore, the effect of the Gly
substitutions on pKa values is highly position-specific; at some positions, a single Gly
substitution can cause large pKa shifts, whereas at others even two Gly substitutions
have only minimal effects.
3.3.2 Thermodynamic stability
The Gly variants were subjected to chemical and acid denaturation to
determine the effects of the substitutions on global stability. Figure 3.3 shows
guanidinium chloride (GdmCl) and acid denaturation curves of ∆+PHS and the Glysubstituted variants. Table 3.2 lists the values of ∆G°, pHmid, and m obtained from
these curves. All of the substitutions were destabilizing, by an amount ranging from
1.2-5 kcal/mol. However, all of the variants had stability of 6.8 kcal/mol or greater.
The loss in stability measured by GdmCl denaturation paralleled the increase in the
pHmid for acid unfolding as expected26. However, the pKa shifts due to the Gly
substitutions do not correlate with either ∆G° or with pHmid. Even in the variants
that showed significant pKa shifts, the majority of Asp and Glu pKa values were
unaffected (Figure 3.3(c)). Furthermore, the double-glycine variants, which were
94
Table 3.2: Stability measured by acid- and GdmCl-induced denaturation.
∆G˚
Protein
pHmida
(kcal/mol)b
m-valueb
∆+PHS
2.25 ± 0.01
11.8 ± 0.05
4.9 ± 0.02
∆+PHS/P11G
2.33 ± 0.01
10.6 ± 0.11
4.7 ± 0.05
∆+PHS/A60G
2.36 ± 0.01
10.1 ± 0.08
4.7 ± 0.04
∆+PHS/A69G
2.61 ± 0.01
9.6 ± 0.18
4.8 ± 0.11
∆+PHS/M98G
2.87 ± 0.01
7.8 ± 0.06
5.4 ± 0.03
∆+PHS/A130G
2.27 ± 0.01
10.9 ± 0.13
4.9 ± 0.06
∆+PHS/A58G/A60G
6.8 ± 0.2
4.6 ± 0.14
∆+PHS/A128G/A130G
8.5 ± 0.13
5.2 ± 0.08
a
Acid denaturation was monitored by Trp fluorescence at 298 K and 100 mM KCl
b
∆G° and m-values measured with guanidine hydrochloride denaturation monitored by
Trp fluorescence at pH 7.0, 298 K, and 100 mM KCl.
95
Figure 3.3: (a) GdnHCl and (b) acid denaturation curves for the proteins used in
this study. Denaturation was monitored by intrinsic Trp fluorescence. Colors
represent the unfolding of ∆+PHS (black), P11G (white), A60G (blue), A69G (red),
M98G (green), and A130G (gray). Lines represent fits to two-state (for GdnHCl) or
three-state (for acid) models as described previously for SNase..26,27 (C) Global
destabilization of ∆+PHS (bars) and pKa shifts (circles) caused by Gly substitutions.
The figure incorporates data from this study and a separately published study
(Doctrow et al., in preparation)
96
among the least stable variants, showed no appreciable shifts in pKa (Figure B.1).
This is consistent with previous work that found no correlation between global
stability and the pKa values of surface histidine residues28.
3.3.3 Crystal structures
Crystal structures were determined for both the A69G and M98G variants, in
both the unliganded forms and in complex with Ca2+ and the inhibitor pdTp.
Crystals of the two ternary complexes were isomorphous with that of ∆+PHS. The
unliganded M98G crystal had a different spacegroup from ∆+PHS, whereas the
unliganded A69G crystal had the same spacegroup as ∆+PHS but different unit cell
dimensions (Table B.2). The aligned Cα traces of ∆+PHS and the A69G and M98G
variants are shown in Figure 3.4. No significant structural changes are visible in any
of the variant structures that could explain the observed pKa shifts. In the ternary
complexes, no significant differences in the protein backbone were observed
between the two glycine variants, or between the glycine variants and ∆+PHS. Nor
were there any differences between the unliganded structures of the two variants.
The conformation of the loop spanning residues 113-117 differed between the
structures of the unliganded and ternary complex forms.
This difference is
consistently observed between structures of nuclease with and without Ca+2 and
pdTp.
Therefore, it is likely that this conformational change results from the
presence or absence of ligands and not from the glycine substitution. The RMSD of
all structures from the ∆+PHS structure was < 0.3 Å for Cα, < 0.4 Å for all heavy
atoms (Table B.3). Except for surface lysine residues, for which electron density is
97
Figure 3.4: Alignment of Cα traces of ∆+PHS (3BDC, white), A69G with (3SR1,
magenta) and without (3T13, yellow) Ca2+ and pdTp, and M98G with (3S9W, green)
and without (3SK8, cyan) Ca2+ and pdTp. Only chain A from 3T13 is shown.
98
often not visible over much of the side chain, no significant differences in side chain
conformations were seen in the vicinity of the Gly substitutions either. The Gly
substitutions do not appear to affect pKa values by changing the overall protein
structure, or at least not by causing a change detectable in a crystal structure.
3.3.4 Hydrogen exchange in Gly variants
Amide hydrogen exchange (HX) rates were measured by NMR in ∆+PHS and
in the A69G and M98G variants to determine the effects of the Gly substitutions on
local conformational fluctuations in solution. These rates are usually interpreted in
terms of the Linderstrøm-Lang scheme29:
Closed
kop
Open
kch
Exchanged
kcl
Under conditions where kch is rate limiting (EX2 regime), the protection factor Pf =
kch/kobs reflects the equilibrium between the closed and open states (lower Pf =
greater population of open states). Residues that are more likely to be in open
states will exchange at rates close to kch and thus will have protection factors close
to 1, whereas residues that are likely to be in closed states will have protection
factors much greater than 1. Therefore, a reduction in the Pf of a residue indicates
an increased population of open states for that residue.
In the A69G variant, significant decreases in Pf are seen in residues 68-71,
comprising the C-terminus of helix 1 and the short loop connecting it to β-strand 4
(α1/β4 loop, Figure 3.5(a)). Asp-95, whose amide forms a hydrogen bond to the
99
Figure 3.5: Significant changes in HX rates observed in the (a) A69G and (b) M98G
variants relative to ∆+PHS. The Gly-substituted residues are shown as spheres.
Residues with measurable exchange rates are colored red, with darker shades
indicating larger increases in rate upon Gly substitution. Black denotes residues
that exchanged within the dead time of the experiment in both the reference and
variant proteins. Residues in white showed no exchange during the course of the
experiment. Gray indicates prolines and any residue whose resonance was not
visible in the HSQC spectrum. Dashed magenta lines indicate hydrogen bonds
whose disruption is consistent with either the observed changes in pKa or the
observed changes in HX rates.
100
carbonyl of Lys-71, also is less protected in the variant. A previous study of HX rates
in SNase suggests that residues in this loop exchange through concerted unfolding
of the loop30. The reduced protection factors for these groups in the variant suggest
that the A69G substitution may promote the unfolding of this loop, thereby favoring
the open state for these residues relative to ∆+PHS.
In the M98G variant the changes in HX are more widespread than in the
A69G variant (Figure 3.5(b)). Lower protection factors are observed in three areas
of the protein: residues 100 and 101 in helix 2 (near the substitution site), residues
125 and 126 in helix 3, and several residues in the large loop spanning residues 7689 (β4/β5 loop). Residues 113-121 and 123 exchanged within the dead time of the
experiment in all variants.
This suggests that the Gly substitution promotes
unfolding of the N-termini of helices 2 and 3 and of the β4/β5 loop. The effect on
the loop was unexpected, given its distance from the substitution (shortest distance
9.8 Å between Met-98-Cγ and Phe-76-HN). The fact that a single substitution affects
the stability of all of these regions indicates that these regions are
thermodynamically coupled to one another. The stability of one region depends on
whether the others are folded, and vice versa.
As noted above, most of the observed pKa shifts are toward the model
compound value.
Since open states resemble model compounds in their high
solvent exposure and lack of hydrogen bonds, our hypothesis would predict that the
residues with normalized pKa values should coincide with regions of reduced Pf. In
both of these variants, the residues with large shifts in pKa were located in or near
regions of reduced Pf (Figure 3.5). Thus, the data are consistent with our hypothesis
101
that the Gly substitutions are shifting the pKa by altering the conformational
ensemble in solution. The exception to this pattern is Glu-101, whose pKa is more
depressed in M98G than in ∆+PHS, even though its Pf is reduced. One possible
explanation is that the substitution might make it more favorable for Glu-101 to
form a hydrogen bond (see Discussion).
3.3.5 15N NMR relaxation measurements
To further probe changes in the dynamics of the protein backbone caused by
Gly substitution,
15N
15N
longitudinal (R1) and transverse (R2) relaxation rates and 1H-
heteronuclear NOE were measured in ∆+PHS and the A69G and M98G variants.
In general, all three parameters were unchanged among the three proteins (Figure
3.6). R1 values tend to be slightly higher across the board in both variants relative to
∆+PHS, suggesting a change in the overall correlation time of the molecule.
Therefore, the Gly substitutions do not appear to affect motions on the timescales
that affect the relaxation parameters (ps-ns, µs-ms).
3.3.6 Structure-based pKa calculations
pKa values for Asp and Glu residues in ∆+PHS and in the A69G and M98G
variants were calculated using several different methods (Figure 3.7(a)).
The
overall correlation between calculated and measured pKa values was comparable
between the variants, but was not particularly good in any of them. Calculations
using PROPKA tended to underestimate the shifts in pKa relative to model
102
Figure 3.6: Backbone 15N R1 (top), R2 (middle), and 1H-15N NOE (bottom) as a
function of residue number for ∆+PHS and the A69G and M98G variants.
103
Figure 3.7: (a) Plot of calculated versus measured pKa values in ∆+PHS (black),
A69G (red), and M98G (green). (b) Plot of calculated versus measured changes in
pKa relative to ∆+PHS for A69G and M98G (same colors as in A). Calculations
performed using FDPB (circles), PROPKA (squares), and MCCE (diamonds) are
included.
104
compound values, whereas in the FDPB calculations many such shifts were
overestimated.
Figure 3.7(b) plots the calculated pKa shifts between the Gly variants and the
background against the measured differences. No correlation is observed for any of
the methods used. The FDPB calculations do a particularly poor job of reproducing
these shifts, with some residues showing a discrepancy of >2 pH units between
calculations and measurements. Both the FDPB and PROPKA calculations are based
solely on the static crystal structure; the failure of these methods implies that the
determinants of the pKa shifts due to Gly substitution are not being captured in the
crystal structure.
MCCE explicitly models the variability and pH-dependent
reorganization of the side chain conformations, but not the backbone. The failure of
MCCE to capture the Gly substitution effects implies that the Gly substitutions do not
affect pKa values only by altering side chain reorganization. Therefore the pKa shifts
in the Gly variants must reflect changes in the backbone reorganization.
3.3.7 COREX calculations
The COREX algorithm31,32 was used to analyze changes in local backbone
stability due to the glycine substitutions. For each variant, changes in the natural
log of the single-residue stability constants relative to ∆+PHS (∆lnKf) were
calculated at pH 7.0. All variants showed significant destabilization (∆lnKf < -1) in
the immediate vicinity of the substitution site (Figure 3.8, top). In addition, residues
36 and 37 were destabilized to varying extents in all variants, with ∆lnKf ranging
105
Figure 3.8: ∆lnKf (top) and ∆lnKp (bottom) relative to ∆+PHS, calculated using
COREX for P11G (black), A60G (blue), A69G (red), M98G (green), and A130G (gray)
at pH 7.0, 298 K. Horizontal dotted lines correspond to ∆lnK = -1, equivalent to ∆∆G
= RT.
106
from ~-0.13 in A130G to ~-3.2 in M98G. The ∆lnKf for these residues correlated
roughly with the change in the measured global stability of the Gly variants,
suggesting that the stability constants of these residues may reflect the global
stability of the protein. Although all of the variants showed significant changes in Kf,
only the variants exhibiting significant pKa shifts showed significant changes in Kp
(Figure 3.8, bottom). This constant, which reflects the probability of a residue being
buried vs. exposed to solvent, determines a residue’s pKa in the COREX algorithm.
The lower the protection constant, the greater the probability of being in a solventexposed environment, and hence the more normal the pKa will be.
Thus the
calculations are consistent with our observation that P11G, A60G, and A130G have
minimal effects on pKa values.
For the A69G variant, aside from residue 36-37, significant destabilization
was observed in residues 61-74 (Figure 3.8, top). This range includes the residues
for which increased HX was observed (68-69 and 71) (Figure 3.5(a)). The other
residues in this region exchanged with rates outside the measureable range in both
background and variant. Hence, changes in stability could not be experimentally
determined for these residues.
Nevertheless, the calculated changes in local
stability and the location of the affected region are qualitatively consistent with the
HX measurements. In addition, there is a large decrease in the calculated protection
constant (Kp) of residue 95 (Figure 3.8, bottom). Thus the large decrease in Kp for
Asp-95 is consistent with the more normal pKa measured for Asp-95 in this variant
(Figure 3.2).
107
M98G showed the largest destabilizations (∆lnKf ~ 3.2) of any of the variants.
Besides residues 36-37, large destabilization was seen in residues 91-106. This
includes the residues with measurable increases in HX rate (92, 100, 101), but also
includes residues whose HX rate does not change measurably (95, 97). Nor is any
large destabilization seen in the other regions with increased HX (residues 76-89
and 125-125) (Figure 3.4(b)). Furthermore, there are no large changes in Kp for any
Asp or Glu residues. The carboxylic group with the largest change in Kp is Asp-95
(∆lnKp = -0.41), whose pKa is unchanged in this variant. The only residue with
|∆lnKp| > 1 is Tyr-93. Thus although COREX does a good job of capturing the effects
of the A69G substitution on the conformational ensemble, it does not adequately
capture the effects of M98G.
3.4 Discussion
The data from this study are consistent with the idea that pKa values of
surface ionizable residues in proteins are governed partly by the inherent
heterogeneity of the protein. Even though Gly substitutions do not add or remove
charges or polar groups, they induced shifts in pKa values similar in magnitude to
what would result from removing a charge 5-10 Å from an ionizable group33.
Crystal structures of the Gly variants showed no evidence that the Gly substitutions
promoted significant conformational changes. It has been shown previously that
Gly substitutions destabilize proteins locally, resulting in increased hydrogen
exchange in residues around the substitution site34,35. In a random coil, Gly can
access more of Φ,Ψ space compared to other amino acids, and thus has greater
108
conformational entropy. Thus, all other factors being equal, Gly will gain more
conformational entropy than other amino acids upon unfolding. Therefore Gly will
favor unfolding more than other amino acids36. Local unfolding may change the
electrostatic environment of an ionizable residue by increasing its solvent exposure,
or by removing hydrogen bonds or Coulomb interactions. Thus, a Gly substitution
may shift the pKa of an ionizable group by increasing the populations of locally
unfolded states. Consistent with this idea, the changes in pKa values seen upon Gly
substitution were accompanied by localized increases in hydrogen exchange rates
(Figure 3.4). Interestingly, the changes in protonation energy reflected in the larger
pKa shifts (∆∆G = 1.36*∆pKa = 0.83-0.84 kcal/mol) are comparable to the measured
value for T*∆∆Sconf upon substitution of alanine with glycine (0.73 ± 0.06 kcal/mol at
T=298 K)36.
15N
relaxation measurements gave no indication that the Gly
substitutions increased backbone motions on the µs-ms timescale, in contrast to
what Beeser et al. observed in BPTI24.
By considering the hydrogen exchange results and the pKa shifts together, we
can understand how the same dynamic processes that give rise to the observed
changes in HX can also produce the observed pKa shifts. In the crystal structure of
∆+PHS, Asp-95 forms hydrogen bonds to the backbone amide groups of Lys-70 and
Lys-71 (Figure 3.5(a)), which depress the pKa of Asp-9518. In the A69G variant, the
pKa of Asp-95 is normalized (Figure 3.2). As explained in Results, the reduced
protection factors in this variant suggest that the Gly substitution promotes
unfolding of the α1/β4 loop that includes residues 70 and 71. This unfolding ought
to break the hydrogen bonds between Asp-95 and the backbone. Therefore, by
109
increasing the unfolded population of the α1/β4 loop, the Gly substitution should
also increase the population of states in which Asp-95 has a more normal pKa. Thus,
the ensemble-averaged pKa should be more normal in A69G compared to ∆+PHS,
which is exactly what is observed experimentally (Figure 3.2).
The case of M98G is more complex. The pH titration data indicate that the
pKa values of Glu-75 and Asp-77 are both normalized in the M98G variant (Figure
3.2). Both of these residues participate in hydrogen bonds to residues in helix-3
that could depress their pKa values: Glu-75 with the His-121 side chain37 and Asp-77
with the Thr-120 side chain and backbone (Figure 3.5(b)). In addition, prior work
has shown that the pKa of His-121, which caps helix 3 and has a depressed pKa in the
background protein37,38, normalizes in the M98G variant28. The pKa shifts of these
three residues (Glu-75, Asp-77, and His-121) are consistent with the increased
unfolding of the helix-3 N-terminus suggested by the HX data. His-121 is likely to be
more solvent exposed when the N-terminus of helix 3 unfolds, and thus will have a
more normal pKa. Unfolding of helix 3 would also break the hydrogen bond between
Glu-75 and His-121, thereby normalizing the pKa of Glu-75. Assuming that Thr-120
also participates in the unfolding of helix 3, then the unfolding would break the
hydrogen bonds between Thr-120 and Asp-77 and normalize the pKa of Asp-77 as
well. Thus by increasing the unfolded population of helix 3, as suggested by the HX
data, the M98G substitution could normalize the pKa values of Glu-75, Asp-77, and
His-121 as observed.
In using Gly substitutions to perturb the pKa values, we assumed that Gly
would promote populations of locally unfolded states in which ionizable residues
110
would have increased exposure to water, thus more normal pKa values.
The
behavior of Glu-101, which became further depressed in M98G, is at odds with this
interpretation. The data suggest that the M98G substitution allows the ionized Glu
to form favorable interactions that are not accessible to it in the background protein.
One possibility is that in the variant, the Glu side chain can adopt a conformation
that allows a hydrogen bond between the side chain and its own backbone amide.
Such a conformation would not be possible in the background protein because of a
steric clash between the Glu-101 and Met-98 side chains. The increased fluctuations
of residues 100-101 indicated by the HX data may facilitate this interaction by
further reducing steric and geometric constraints. The Glu-101 backbone amide
does not have a hydrogen-bonding partner in the background protein. The crystal
structure of M98G shows Glu-101 in the same conformation as in the background
protein, suggesting that the dominant conformation of Glu-101 is the same in both
proteins.
However, the proposed hydrogen-bonded conformation may still be
present in a small but significant population in M98G. As long as this population is
larger than in ∆+PHS, the ensemble-averaged pKa will be more depressed in the
variant compared to the background.
Our results demonstrate that the pKa value of an ionizable group can be
shifted by as many as 0.6 pH units (and possibly more in the case of Asp-77) by a
single amino acid substitution that does not affect either the polarity or the number
of charges in the protein. The results also suggest that the effect involves increased
exposure of the ionizable moiety to bulk water and that this is a consequence of the
increase in the propensity for fluctuation of the backbone into locally unfolded
111
states.
These results have important implications for structure-based pKa
calculations. The calculated pKa values in Figure 3.7 were obtained using various
methods (FDPB, PROPKA, MCCE). None of these methods is capable of reproducing
the consequences of Gly substitutions on the pKa of carboxyl groups. All of the
calculations predict large pKa shifts for residues that are unaffected by the
substitution, while predicting no pKa shifts for the residues that are affected. Even
in cases where the calculations correctly identify residues whose pKa values are
shifted by the Gly substitution, the direction of the shift is usually in the wrong
direction. Because the error in the calculated ∆pKa is not systematic, the agreement
between calculated and measured ∆pKa values cannot be improved by arbitrarily
adjusting the protein dielectric constant.
Two of the pKa calculation methods tested here (FDPB and PROPKA) use a
static structure to represent the protein. The third, MCCE, includes side chain
heterogeneity explicitly while keeping the backbone static. None of these methods
are capable of explicitly reproducing the effects of the Gly substitution on backbone
reorganization or local unfolding, and must rely on implicit treatment of these
effects through dielectric constant. However, the experimental data suggest that the
Gly substitutions have a significant effect on backbone reorganization, and that this
effect is not uniform throughout the protein. Therefore it is impossible to treat
implicitly the effect of the Gly substitutions using a single dielectric constant for the
entire protein. The backbone dynamics, and local unfolding in particular, must be
treated explicitly in calculations.
This has been attempted using molecular
112
dynamics (MD) simulations20, but these cannot adequately sample the timescales
relevant to backbone reorganization14,15.
The COREX algorithm provides another way to treat backbone dynamics in
calculations. Because COREX generates its ensemble through enumeration of states,
rather than dynamic sampling, it is not limited in the range of timescales that it can
model. The coupling between local unfolding and side chain ionization is treated
explicitly by assigning separate pKa values to unfolded versus folded residues, and
then averaging the protonation state over the entire ensemble (equation (1)). This
model was able to accurately reproduce the acid unfolding of SNase, and to identify
the carboxylic residues responsible for this unfolding23. Figure 3.8 shows that
COREX is able to reproduce the measured effects of the A69G substitution on local
stability (HX) and on the pKa of Asp-95. This suggests that the COREX model
adequately describes the effect of this substitution on the protein, and that the shift
in the pKa of Asp-95 in this variant results from an increased population of states in
which Asp-95 titrates with a more normal pKa.
However, COREX does not reproduce the effects of the M98G substitution on
either local stability or pKa values. In this variant, the pKa of Glu-101 becomes more
depressed. For COREX to reproduce this, the residue’s environment would have to
become more stable in the variant. This conflicts with the HX results, which show
that the M98G substitution destabilizes Glu-101. We proposed above that the pKa of
Glu-101 is more depressed in M98G because it can form a hydrogen bond that is not
allowed in the native state. If this is correct, then COREX cannot reproduce the pKa
shift of Glu-101, as COREX does not permit non-native hydrogen bonds to form.
113
COREX also does not reproduce the measured pKa shifts of Glu-75, Asp-77, and His121. These residues are all part of a network of hydrogen bonds37, which could
energetically couple regions at opposite ends of the network. Because of this
coupling, if one of these regions (e.g. helix 2) is destabilized by the M98G
substitution, all other regions connected to this network (e.g. helix 3, β4/β5 loop)
will be destabilized39. Consistent with this interpretation, the HX data show that
M98G destabilizes helix 3 and the β4/β5 loop in addition to helix 2 (Figure 3.5(b)).
Apparently COREX is unable to reproduce the energetic coupling between these
regions, and therefore cannot reproduce the effects of the M98G substitution on
local stabilities. Most likely COREX would have an equally difficult time with any
other substitution that perturbs this h-bond network.
3.5 Conclusion
This study provides strong experimental evidence that local backbone
reorganization influences pKa values in proteins. Substitutions to Gly that did not
change the charge or polarity of amino acids shifted the pKa values of Asp and Glu
residues in SNase. The substitutions did not affect the crystal structure of the
protein; this is clear evidence of ways in which crystal structures do not reflect all of
the determinants of pKa values. However, the substitutions with Gly did change HX
rates, consistent with the substitutions promoting local unfolding. Local unfolding
can change an ionizable residue’s average environment, thus shifting the pKa. The
changes in HX occured in the same parts of the protein where the pKa shifts occur,
further suggesting a connection between pKa values and local unfolding.
114
Many commonly used algorithms for structure-based pKa calculations
represent the protein by the crystal structure alone. The results presented here
illustrate one way in which such a representation is inadequate; calculations based
on a single, static protein structure were unable to reproduce the pKa shifts caused
by Gly substitutions. Calculations that only allow the side chains to reorganize were
similarly ineffective. These results, combined with our experimental measurements,
indicate that structure-based pKa calculations must take into account the full
ensemble of backbone conformations in solution in order to be accurate and useful.
Developing an algorithm that can adequately sample this ensemble within a
practical amount of computation time remains a challenge40. Considerable effort
has been put into developing constant-pH molecular dynamics (CPHMD) methods,
which explicitly couple backbone dynamics with changes in protonation states41–51.
If successful, such methods would represent a major advance in the field of protein
electrostatics and could make great contributions to understanding protein
evolution and protein engineering. The effects of Gly substitutions on pKa values
would provide a useful benchmark for further calibrating these methods.
3.6 Materials and methods
3.6.1 Site directed mutagenesis and protein purification
All mutations were made in a highly stable variant of SNase known as ∆+PHS,
which differs from wild-type SNase by five substitutions (G50F, V51N, P117G,
H124L, and S128A) and a truncation (residues 44-49). Throughout this paper,
115
residue numbers refer to the position in the wild-type sequence. Mutations were
engineered in the pET24a+ plasmid and expressed in E. coli BL21(DE3) cells.
Proteins were expressed and purified according to the procedure of Shortle and
Meeker52. For NMR experiments, uniformly
15N-
or
made by growing the cells in M9 minimal media with
13C6-D-glucose
13C/15N-labeled
protein was
15NH4Cl
15NH4Cl
or with
and
as described previously18,37 and purified according to the same
procedure as for the unlabeled protein.
3.6.2 Equilibrium thermodynamics
The thermodynamic stability of the Gly-substituted variants was determined
by denaturation with guanidinium chloride (GdmCl) and with acid, monitored by
the intrinsic fluorescence of Trp-140 (λex = 296 nm, λem = 326 nm) as described
previously26.
Experiments were performed with an AVIV ATF-105 or 107
automated fluorometer (Aviv Biomedical Inc. Lakewood, NJ) equipped with a
Hamilton MicroLab 531C automated titration pump. All data were collected at 298
K and 100 mM KCl.
GdmCl denaturation was carried out at pH 7.0.
GdmCl
denaturation experiments were analyzed by non-linear least squares fitting to a
two-state model to obtain the standard Gibbs energy of unfolding (∆G°H2O), as
described previously26. Acid denaturation experiments were analyzed by non-linear
least squares fitting to a three-state model to obtain the midpoint of the unfolding
transition (pHmid), as described previously27. For acid unfolding experiments, only
the pHmid corresponding to global unfolding is reported.
116
3.6.3 NMR spectroscopy
Uniformly
15N-
or
13C/15N-labeled
samples were prepared by exchanging
from H2O into aqueous buffer containing 100 mM KCl, 0.5 mM NaN3, and 10% D2O
(v/v) by successive dilution in Amicon Ultra-4 tubes (Millipore). Final protein
concentration ranged from 0.7 to 1.1 mM. All NMR experiments were collected at
298 K on either a Bruker Avance or Avance II 600 MHz spectrometer. H N and N
resonances in the A69G and M98G variants were assigned using standard
HNCACB53, CBCACONH54, and C-C TOCSY (CO)NH55 triple-resonance NMR
experiments. The sample buffer for assignments contained 25 mM acetate buffer
(pH 4.7) in addition to the components listed above. Acetate buffer was prepared by
mixing potassium acetate and glacial acetic acid in amounts calculated to yield the
desired pH according to the Henderson-Hasselbalch equation. The final sample pH
was 4.76 for A69G and 4.69 for M98G. All spectra were processed using NMRPipe56
and analyzed using Sparky57.
Asp and Glu pKa values were measured on a Bruker Avance or Avance II 600
MHz NMR spectrometer with a cryogenic-TCI probe (with a cryocooled
13C
preamplifier). The Cγ and Cδ resonances were used to monitor the titration of Asp
and Glu resonances, respectively. These resonances have been assigned previously
in ∆+PHS18 and were measured using a
13C-detected
CBCGCO experiment18. The
resonances in the spectra of the variants could be assigned by comparison with the
∆+PHS spectrum. pKa values were obtained from least squares fitting to a one- or
two-site modified Hill equation, as previously described in Section 2.6.3.
117
Samples for HX measurements were prepared by lyophilizing uniformly 15Nlabeled protein. 1H to 2H exchange was initiated by dissolving lyophilized protein in
D2O buffer containing 25 mM acetate (pH* 4.8), 100 mM KCl, and 0.5 mM NaN3, to a
final protein concentration of 1 mM. The uncorrected sample pH, measured at the
end of the exchange measurements, ranged from 5.05-5.13.
Exchange was
monitored in real time by sequential 2D HSQC experiments, separated by
progressively longer intervals.
HX rates were obtained by fitting a single-exponential decay function to the
peak heights as a function of time, using the R statistics package58. The peaks of
residues 90 and 122 overlap in the spectra of ∆+PHS and A69G, as do residues 66
and 86 in A69G. However, in both cases the two overlapping residues exchange at
very different rates, resulting in a bi-exponential decay profile for the single
observed peak. By fitting a bi-exponential decay function to this peak, the exchange
rates for both residues can be resolved. Based on the behavior of the neighboring
residues, residues 122 and 86 were assigned the faster rates.
(Table B.4).
Protection factors (Pf) were calculated as the ratio kch/kobs, where kobs is the rate
obtained from the exponential fit, and kch is the intrinsic rate calculated from the
sequence and the experimental conditions according to the method of Bai et al.59
Following Skinner et al.30 the reference acid- and base-catalyzed rate constants for
Asp and Glu residues were increased by a factor of 2.5 to account for their known
systematic deviation from the values predicted by Bai et al.60
Many residues showed minimal exchange over the duration of the
experiment, which ranged from 45 to 93 hours. For these residues, the fitted rate
118
constants were used only if the fitting program returned a p-value less than 0.0005.
Otherwise, kobs was assigned an upper limit equal to –ln(0.9)/tmax, where tmax is the
last measured time point. This corresponds to the rate for which a residue would
have exchanged by 10% by the end of the experiment. A lower limit for Pf was then
calculated from this upper limit and the calculated value of kch. Protection factors
for residues that completely exchanged within the first three time points were
assigned upper limits, using the fastest measured rate in the variant as the lower
limit for kobs. This was only done for residues whose exchange rate could be
measured in at least one variant. Residues whose exchange rate was too fast to
measure in all proteins were excluded from analysis.
Experiments to measure R1, R2, and the heteronuclear NOE of backbone
15N
atoms in the Gly variants were conducted using established two-dimensional
heteronuclear correlation experiments61,62.
The pulse sequence for R2
measurements incorporated duty-cycle heating compensation63.
uniformly
15N-labeled
Samples of
protein were prepared in the same manner as for the
assignment experiments. For R1 measurements, the maximum relaxation delay was
1.1 s (A69G) or 1.5 s (M98G). For R2 measurements, the maximum delay was 106
ms (A69G) or 144 ms (M98G). 6-9 time points were collected for all measurements.
One randomly selected time point from each measurement was collected twice for
error estimation.
Relaxation rates were obtained by fitting 1H-15N cross-peak
heights versus time to an exponential decay function using the CurveFit program
from the laboratory of Dr. Arthur Palmer III (www.palmer.hs.columbia.edu).
Steady-state NOEs were calculated as the ratio of the 1H-15N cross peak heights in
119
the presence and absence of proton presaturation. The peak heights with and
without presaturation were each calculated as the average over three experiments.
3.6.4 X-ray crystallography
Crystals of the A69G and M98G variants were grown at 277 K using the
hanging-drop, vapor diffusion method. Crystals were grown both in the presence
and absence of Ca2+ and the inhibitor thymidine-3’,5’-bis-phosphate (pdTp).
Crystals of the M98G variant with Ca2+ and pdTp were grown in a solution
containing 20% (v/v) 2-methyl-2,4-pentanediol (MPD) and 25 mM potassium
phosphate buffer, pH 7.0, whereas crystals without Ca2+ and pdTp were grown in a
solution containing 34% (v/v) MPD and 25 mM potassium phosphate buffer, pH 7.0.
Crystals of the A69G variant with Ca2+ and pdTp were grown in a solution
containing 25% (v/v) MPD and 25 mM potassium phosphate buffer, pH 9.0, whereas
crystals without Ca2+ and pdTp were grown in a solution containing 41% (v/v) MPD,
25 mM potassium phosphate buffer, pH 7.0. The initial protein concentration was
11.8 mg/mL for M98G and 13.0 mg/mL for A69G. For crystallization with Ca 2+ and
pdTp, the protein was mixed with 3 M equiv. CaCl2 and 2 M equiv. pdTp. The
protein or protein/CaCl2/pdTp mixture was then mixed with the reservoir solution
in a 1:1 ratio to form the hanging drop. For data collection, crystals were suspended
with mother liquor in a cryoloop and flash-cooled in liquid nitrogen. All proteins
crystallized in spacegroup P21, except for M98G in the absence of Ca2+ and pdTp,
which crystallized in spacegroup P41.
120
Data were collected from a single crystal of each variant on beamline X-25 at
the National Synchrotron Light Source at Brookhaven National Laboratory.
Reflections were indexed, integrated, scaled and merged using HKL200064. Phases
for all structures were determined by molecular replacement using the Phaser65
program within the CCP4 suite66. The structure of ∆+PHS (PDB accession code
3BDC) with the mutated residue truncated to glycine, all waters removed, and all Bfactors set to 20.0 Å2 was used as a search model. Alternating rounds of structure
refinement with Refmac567 and model building with Coot68 yielded the final models.
TLS refinement69,70 was used during the later rounds of refinement. Geometries of
the final models were evaluated using the MolProbity server71,72.
Both of the M98G models and the A69G model with Ca2+ and pdTp include
residues 7-141 and have one protein per asymmetric unit. The A69G model without
Ca2+ and pdTp has two proteins per asymmetric unit, one of which includes residues
6-141 and the other of which includes residues 7-141. The models with Ca2+ and
pdTp contained one Ca2+ and one pdTp each. The model of M98G without Ca2+ and
pdTp contained two phosphates and one MPD molecule, whereas the model of A69G
without Ca2+ and pdTp contained three phosphates and four MPD molecules. The
latter model also contains one Ca2+, even though no CaCl2 was added to the protein
used to grow the crystal. The electron density associated with this Ca2+ is too strong
to be from a water molecule, and is located where Ca2+ normally binds to the
protein. The Ca2+ may have come from trace calcium contamination in the well
where the crystal was grown. No Ca2+ appears to be bound to the second molecule
121
in the asymmetric unit. Data collection and refinement statistics are summarized in
Table B.1.
3.6.5 Calculations
All structure-based pKa calculations were performed using the crystal
structures of ∆+PHS (PDB accession code 3BDC), ∆+PHS/A69G (3SR1), or
∆+PHS/M98G (3S9W). Ca2+ and pdTp are bound to the protein in all of these
structures, but were deleted from the structure prior to running the calculations.
Structures were not relaxed prior to pKa calculations.
All calculations were
performed at 298 K.
Finite difference Poisson-Boltzmann (FDPB) calculations with a static
structure were performed using the finite difference Poisson-Boltzmann algorithm
within the University of Houston Brownian Dynamics package73,74 using the fullcharge implementation, as described previously for SNase15. Calculations used a
protein dielectric constant of 10 and 100 mM ionic strength. Charges from the
PARSE parameter set were used75. For the histidines, hydrogen atoms were placed
on Nε2 of His-8 and Nδ1 of His-121.
This gave the best agreement with
experimental pKa values.
pKa values were also calculated with PROPKA version 3.1 on the PROPKA
web interface (propka.ki.ku.dk)13,76,77. The effects of side chain conformational
variability
were
also
explored
using
the
multi-conformation
electrostatics (MCCE) algorithm12,21,78, version 2.5.
The parameters of the
calculations were those used for previous calculations on SNase79.
122
continuum
The FULL
method of conformer generation was used to explore all possible side chain
rotamers. Electrostatic interactions were calculated using a dielectric constant of 10
and a salt concentration of 0.15 M. Lennard-Jones interactions were scaled by a
factor of 0.25.
COREX calculations were performed using source code provided by Dr.
Steven Whitten (Texas State University-San Marcos)31.
Calculations were
performed on 3BDC and models of the A69G and M98G variants generated from
3BDC by truncating residue 69 or 98 to glycine in silico. Models of the variants were
used instead of crystal structures to ensure that any calculated changes were solely
the result of the Gly substitution, and not to any small changes in the coordinates of
the rest of the protein. The size of the folding units was 8 residues, which resulted
in ~6.5 x 105 microstates in the ensemble for each protein.
The pKa values
calculated for ∆+PHS using the FDPB procedure described above were used as the
native state pKa values in all COREX calculations. The solvent accessibility cutoff for
assignment of native versus unfolded pKa values was 0.45 for histidines and 0.31 for
all other residues. These are the same values used previously for wild-type SNase
calculations23,80.
Entropy-scaling factors were chosen to reproduce the
experimentally measured free energy of unfolding of the SNase variants at pH 7.0,
and ranged from 0.97-0.981.
123
3.7 References
1. Warshel, A. (2003). Computer Simulations of Enzyme Catalysis: Methods,
Progress, and Insights. Annual Review of Biophysics and Biomolecular Structure
32, 425–443
2. Burykin, A. & Warshel, A. (2003). What Really Prevents Proton Transport
through Aquaporin? Charge Self-Energy versus Proton Wire Proposals.
Biophysical Journal 85, 3696–3706
3. Burykin, A. & Warshel, A. (2004). On the origin of the electrostatic barrier for
proton transport in aquaporin. FEBS Letters 570, 41–46
4. Braun-Sand, S., Strajbl, M. & Warshel, A. (2004). Studies of Proton Translocations
in Biological Systems: Simulating Proton Transport in Carbonic Anhydrase by
EVB-Based Models. Biophysical Journal 87, 2221–2239
5. Chu, A.H., Turner, B.W. & Ackers, G.K. (1984). Effects of protons on the
oxygenation-linked subunit assembly in human hemoglobin. Biochemistry 23,
604–617
6. Ehrlich, L.S., Liu, T., Scarlata, S., Chu, B. & Carter, C.A. (2001). HIV-1 Capsid
Protein Forms Spherical (Immature-Like) and Tubular (Mature-Like) Particles
in Vitro: Structure Switching by pH-induced Conformational Changes.
Biophysical Journal 81, 586–594
7. Tanford, C. & Kirkwood, J.G. (1957). Theory of Protein Titration Curves. I.
General Equations for Impenetrable Spheres. J. Am. Chem. Soc. 79, 5333–5339
8. Matthew, J.B., Gurd, F.R.N., Garcia-Moreno, B.E., Flanagan, M.A., March, K.L. &
Shire, S.J. (1985). pH-Dependent Processes in Protein. Critical Reviews in
Biochemistry and Molecular Biology 18, 91–197
9. Klapper, I., Hagstrom, R., Fine, R., Sharp, K. & Honig, B. (1986). Focusing of
electric fields in the active site of Cu-Zn superoxide dismutase: Effects of ionic
strength and amino-acid modification. Proteins: Structure, Function, and
Bioinformatics 1, 47–59
10. Bashford, D. & Karplus, M. (1990). pKa’s of ionizable groups in proteins: atomic
detail from a continuum electrostatic model. Biochemistry 29, 10219–10225
11. Antosiewicz, J., McCammon, J.A. & Gilson, M.K. (1994). Prediction of Phdependent Properties of Proteins. Journal of Molecular Biology 238, 415–436
12. Alexov, E.G. & Gunner, M.R. (1997). Incorporating protein conformational
flexibility into the calculation of pH-dependent protein properties. Biophysical
Journal 72, 2075–2093
13. Li, H., Robertson, A.D. & Jensen, J.H. (2005). Very fast empirical prediction and
rationalization of protein pKa values. Proteins: Structure, Function, and
Bioinformatics 61, 704–721
14. Warshel, A., Sharma, P.K., Kato, M. & Parson, W.W. (2006). Modeling electrostatic
effects in proteins. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics
1764, 1647–1676
15. Fitch, C.A., Whitten, S.T., Hilser, V.J. & García‐ Moreno E., B. (2006). Molecular
mechanisms of pH‐ driven conformational transitions of proteins: Insights from
124
continuum electrostatics calculations of acid unfolding. Proteins: Structure,
Function, and Bioinformatics 63, 113–126
16. Forsyth, W.R., Antosiewicz, J.M. & Robertson, A.D. (2002). Empirical
relationships between protein structure and carboxyl pKa values in proteins.
Proteins: Structure, Function, and Genetics 48, 388–403
17. Edgcomb, S.P. & Murphy, K.P. (2002). Variability in the pKa of histidine sidechains correlates with burial within proteins. Proteins: Structure, Function, and
Bioinformatics 49, 1–6
18. Castañeda, C.A., Fitch, C.A., Majumdar, A., Khangulov, V., Schlessman, J.L. &
García‐ Moreno, B.E. (2009). Molecular determinants of the pKa values of Asp
and Glu residues in staphylococcal nuclease. Proteins: Structure, Function, and
Bioinformatics 77, 570–588
19. García-Moreno E, B. & Fitch, C.A. (2004). Structural Interpretation of pH and
Salt-Dependent Processes in Proteins with Computational Methods. Methods in
Enzymology 380, 20–51
20. Van Vlijmen, H.W.T., Schaefer, M. & Karplus, M. (1998). Improving the accuracy
of protein pKa calculations: Conformational averaging versus the average
structure. Proteins: Structure, Function, and Bioinformatics 33, 145–158
21. Georgescu, R.E., Alexov, E.G. & Gunner, M.R. (2002). Combining conformational
flexibility and continuum electrostatics for calculating pKas in proteins.
Biophysical Journal 83, 1731–1748
22. Hilser, V.J., García-Moreno E., B., Oas, T.G., Kapp, G. & Whitten, S.T. (2006). A
Statistical Thermodynamic Model of the Protein Ensemble. Chemical Reviews
106, 1545–1558
23. Whitten, S.T., García-Moreno E., B. & Hilser, V.J. (2005). Local conformational
fluctuations can modulate the coupling between proton binding and global
structural transitions in proteins. Proceedings of the National Academy of
Sciences of the United States of America 102, 4282–4287
24. Beeser, S.A., Goldenberg, D.P. & Oas, T.G. (1997). Enhanced protein flexibility
caused by a destabilizing amino acid replacement in BPTI. Journal of Molecular
Biology 269, 154–164
25. Maity, H., Rumbley, J.N. & Englander, S.W. (2006). Functional role of a protein
foldon—An Ω‐ loop foldon controls the alkaline transition in ferricytochrome c.
Proteins: Structure, Function, and Bioinformatics 63, 349–355
26. Whitten, S.T. & García-Moreno E., B. (2000). pH dependence of stability of
staphylococcal nuclease: Evidence of substantial electrostatic interactions in the
denatured state. Biochemistry 39, 14292–14304
27. Karp, D.A., Gittis, A.G., Stahley, M.R., Fitch, C.A., Stites, W.E. & García-Moreno E., B.
(2007). High Apparent Dielectric Constant Inside a Protein Reflects Structural
Reorganization Coupled to the Ionization of an Internal Asp. Biophysical Journal
92, 2041–2053
28. Doctrow, B.D., Baran, K.L., Chimenti, M.S., Herbst, K.J., Fitch, C.A., Majumdar, A. &
Garcia-Moreno E, B. Local flexibility as a determinant of pKa values of surface
ionizable groups in proteins. In preparation
125
29. Hvidt, A. & Nielsen, S.O. (1966). Hydrogen Exchange in Proteins. Advances in
Protein Chemistry 21, 287–386
30. Skinner, J.J., Lim, W.K., Bédard, S., Black, B.E. & Englander, S.W. (2012). Protein
dynamics viewed by hydrogen exchange. Protein Science 21, 996–1005
31. Hilser, V.J. & Freire, E. (1996). Structure-based Calculation of the Equilibrium
Folding Pathway of Proteins. Correlation with Hydrogen Exchange Protection
Factors. Journal of Molecular Biology 262, 756–772
32. Hilser, V.J. & Freire, E. (1997). Predicting the equilibrium protein folding
pathway: Structure-based analysis of staphylococcal nuclease. Proteins:
Structure, Function, and Bioinformatics 27, 171–183
33. Lee, K.K., Fitch, C.A. & García-Moreno E., B. (2002). Distance dependence and salt
sensitivity of pairwise, coulombic interactions in a protein. Protein Science 11,
1004–1016
34. Huyghues-Despointes, B.M.P., Langhorst, U., Steyaert, J., Pace, C.N. & Scholtz, J.M.
(1999). Hydrogen-Exchange Stabilities of RNase T1 and Variants with Buried
and Solvent-Exposed Ala → Gly Mutations in the Helix. Biochemistry 38, 16481–
16490
35. Maity, H., Lim, W.K., Rumbley, J.N. & Englander, S.W. (2003). Protein hydrogen
exchange mechanism: Local fluctuations. Protein Science 12, 153–160
36. D’Aquino, J.A., Gómez, J., Hilser, V.J., Lee, K.H., Amzel, L.M. & Freire, E. (1996). The
magnitude of the backbone conformational entropy change in protein folding.
Proteins: Structure, Function, and Bioinformatics 25, 143–156
37. Baran, K.L., Chimenti, M.S., Schlessman, J.L., Fitch, C.A., Herbst, K.J. & GarcíaMoreno, B. (2008). Electrostatic effects in a network of polar and ionizable
groups in staphylococcal nuclease. Journal of Molecular Biology 379, 1045–1062
38. Castaneda, C.A. (2009). Determinants of electrostatic energies and pKa values in
proteins. at
<http://search.proquest.com/docview/304907798?accountid=11752>
39. Hilser, V.J. & Thompson, E.B. (2007). Intrinsic disorder as a mechanism to
optimize allosteric coupling in proteins. Proceedings of the National Academy of
Sciences of the United States of America 104, 8311–8315
40. Alexov, E., Mehler, E.L., Baker, N., M. Baptista, A., Huang, Y., Milletti, F., Erik
Nielsen, J., Farrell, D., Carstensen, T., Olsson, M.H.M., Shen, J.K., Warwicker, J.,
Williams, S. & Word, J.M. (2011). Progress in the prediction of pKa values in
proteins. Proteins: Structure, Function, and Bioinformatics 79, 3260–3275
41. Mongan, J. & Case, D.A. (2005). Biomolecular simulations at constant pH. Current
Opinion in Structural Biology 15, 157–163
42. Mongan, J., Case, D.A. & McCammon, J.A. (2004). Constant pH molecular
dynamics in generalized Born implicit solvent. Journal of Computational
Chemistry 25, 2038–2048
43. Baptista, A.M., Martel, P.J. & Petersen, S.B. (1997). Simulation of protein
conformational freedom as a function of pH: constant-pH molecular dynamics
using implicit titration. Proteins: Structure, Function, and Bioinformatics 27, 523–
544
126
44. Lee, M.S., Salsbury, F.R. & Brooks, C.L. (2004). Constant-pH molecular dynamics
using continuous titration coordinates. Proteins: Structure, Function, and
Bioinformatics 56, 738–752
45. Donnini, S., Tegeler, F., Groenhof, G. & Grubmü ller, H. (2011). Constant pH
Molecular Dynamics in Explicit Solvent with λ-Dynamics. Journal of Chemical
Theory and Computation 7, 1962–1978
46. Khandogin, J. & Brooks, C.L. (2006). Toward the Accurate First-Principles
Prediction of Ionization Equilibria in Proteins†. Biochemistry 45, 9363–9373
47. Meng, Y. & Roitberg, A.E. (2010). Constant pH Replica Exchange Molecular
Dynamics in Biomolecules Using a Discrete Protonation Model. Journal of
Chemical Theory and Computation 6, 1401–1412
48. Williams, S.L., de Oliveira, C.A.F. & McCammon, J.A. (2010). Coupling Constant pH
Molecular Dynamics with Accelerated Molecular Dynamics. Journal of Chemical
Theory and Computation 6, 560–568
49. Itoh, S.G., Damjanović, A. & Brooks, B.R. (2011). pH replica-exchange method
based on discrete protonation states. Proteins: Structure, Function, and
Bioinformatics 79, 3420–3436
50. Wallace, J.A. & Shen, J.K. (2011). Continuous Constant pH Molecular Dynamics in
Explicit Solvent with pH-Based Replica Exchange. Journal of Chemical Theory and
Computation 7, 2617–2629
51. Goh, G.B., Hulbert, B.S., Zhou, H. & Brooks, C.L. (2014). Constant pH molecular
dynamics of proteins in explicit solvent with proton tautomerism. Proteins:
Structure, Function, and Bioinformatics [Online early access],
http://onlinelibrary.wiley.com/doi/10.1002/prot.24499/abstract
52. Shortle, D. & Meeker, A.K. (1986). Mutant forms of staphylococcal nuclease with
altered patterns of guanidine hydrochloride and urea denaturation. Proteins:
Structure, Function, and Genetics 1, 81–89
53. Wittekind, M. & Mueller, L. (1993). HNCACB, a High-Sensitivity 3D NMR
Experiment to Correlate Amide-Proton and Nitrogen Resonances with the
Alpha- and Beta-Carbon Resonances in Proteins. Journal of Magnetic Resonance,
Series B 101, 201–205
54. Grzesiek, S. & Bax, A. (1992). Correlating backbone amide and side chain
resonances in larger proteins by multiple relayed triple resonance NMR. J. Am.
Chem. Soc. 114, 6291–6293
55. Grzesiek, S., Anglister, J. & Bax, A. (1993). Correlation of Backbone Amide and
Aliphatic Side-Chain Resonances in 13C/15N-Enriched Proteins by Isotropic
Mixing of 13C Magnetization. Journal of Magnetic Resonance, Series B 101, 114–
119
56. Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. & Bax, A. (1995).
NMRPipe: A multidimensional spectral processing system based on UNIX pipes.
Journal of Biomolecular NMR 6, 277–293
57. Goddard, T.D. & Kneller, D.G. (University of California, San Francisco: ).SPARKY 3.
58. R Development Core Team (R Foundation for Statistical Computing: Vienna,
Austria, 2009). R: A language and environment for statistical computing. at
<http://www.R-project.org>
127
59. Bai, Y., Milne, J.S., Mayne, L. & Englander, S.W. (1993). Primary structure effects
on peptide group hydrogen exchange. Proteins: Structure, Function, and
Bioinformatics 17, 75–86
60. Mori, S., van Zijl, P.C.M. & Shortle, D. (1997). Measurement of water–amide
proton exchange rates in the denatured state of staphylococcal nuclease by a
magnetization transfer technique. Proteins: Structure, Function, and
Bioinformatics 28, 325–332
61. Kay, L.E., Torchia, D.A. & Bax, A. (1989). Backbone dynamics of proteins as
studied by nitrogen-15 inverse detected heteronuclear NMR spectroscopy:
application to staphylococcal nuclease. Biochemistry 28, 8972–8979
62. Loria, J.P., Rance, M. & Palmer, A.G. (1999). A Relaxation-Compensated
Carr−Purcell−Meiboom−Gill Sequence for Characterizing Chemical Exchange by
NMR Spectroscopy. J. Am. Chem. Soc. 121, 2331–2332
63. Yip, G.N.B. & Zuiderweg, E.R.P. (2005). Improvement of duty-cycle heating
compensation in NMR spin relaxation experiments. Journal of Magnetic
Resonance 176, 171–178
64. Otwinowski, Z. & Minor, W. (1997). Processing of X-ray diffraction data collected
in oscillation mode. Methods in Enzymology 276, 307–326
65. McCoy, A.J., Grosse-Kunstleve, R.W., Storoni, L.C. & Read, R.J. (2005). Likelihoodenhanced fast translation functions. Acta Crystallographica Section D-Biological
Crystallography 61, 458–464
66. Bailey, S. (1994). The CCP4 suite: programs for protein crystallography. Acta
Crystallographica Section D-Biological Crystallography 50, 760–763
67. Murshudov, G.N., Vagin, A.A. & Dodson, E.J. (1997). Refinement of
macromolecular structures by the maximum-likelihood method. Acta
Crystallographica Section D-Biological Crystallography 53, 240–255
68. Emsley, P. & Cowtan, K. (2004). Coot: model-building tools for molecular
graphics. Acta Crystallographica Section D-Biological Crystallography 60, 2126–
2132
69. Painter, J. & Merritt, E.A. (2006). Optimal description of a protein structure in
terms of multiple groups undergoing TLS motion. Acta Crystallographica Section
D-Biological Crystallography 62, 439–450
70. Painter, J. & Merritt, E.A. (2006). TLSMD web server for the generation of multigroup TLS models. Journal of Applied Crystallography 39, 109–111
71. Chen, V.B., Arendall, W.B., Headd, J.J., Keedy, D.A., Immormino, R.M., Kapral, G.J.,
Murray, L.W., Richardson, J.S. & Richardson, D.C. (2009). MolProbity: all-atom
structure validation for macromolecular crystallography. Acta Crystallographica
Section D-Biological Crystallography 66, 12–21
72. Davis, I.W., Leaver-Fay, A., Chen, V.B., Block, J.N., Kapral, G.J., Wang, X., Murray,
L.W., Arendall, W.B., Snoeyink, J., Richardson, J.S. & Richardson, D.C. (2007).
MolProbity: all-atom contacts and structure validation for proteins and nucleic
acids. Nucleic Acids Research 35 Suppl 2, W375–W383
73. Davis, M.E., Madura, J.D., Luty, B.A. & McCammon, J.A. (1991). Electrostatics and
diffusion of molecules in solution: simulations with the University of Houston
Brownian dynamics program. Computer Physics Communications 62, 187–197
128
74. Madura, J.D., Briggs, J.M., Wade, R.C., Davis, M.E., Luty, B.A., Ilin, A., Antosiewicz,
J., Gilson, M.K., Bagheri, B., Scott, L.R. & McCammon, J.A. (1995). Electrostatics
and diffusion of molecules in solution: simulations with the University of
Houston Brownian Dynamics program. Computer Physics Communications 91,
57–95
75. Sitkoff, D., Sharp, K.A. & Honig, B. (1994). Accurate calculation of hydration free
energies using macroscopic solvent models. The Journal of Physical Chemistry
98, 1978–1988
76. Olsson, M.H.M., Søndergaard, C.R., Rostkowski, M. & Jensen, J.H. (2011).
PROPKA3: Consistent treatment of internal and surface residues in empirical
pKa predictions. Journal of Chemical Theory and Computation 7, 525–537
77. Søndergaard, C.R., Olsson, M.H.M., Rostkowski, M. & Jensen, J.H. (2011).
Improved Treatment of Ligands and Coupling Effects in Empirical Calculation
and Rationalization of pKa Values. Journal of Chemical Theory and Computation
7, 2284–2295
78. Song, Y., Mao, J. & Gunner, M.R. (2009). MCCE2: Improving protein pKa
calculations with extensive side chain rotamer sampling. Journal of
Computational Chemistry 30, 2231–2247
79. Gunner, M.R., Zhu, X. & Klein, M.C. (2011). MCCE analysis of the pKas of
introduced buried acids and bases in staphylococcal nuclease. Proteins:
Structure, Function, and Bioinformatics 79, 3306–3319
80. Whitten, S.T., García‐ Moreno, B.E. & Hilser, V.J. (2008). Ligand Effects on the
Protein Ensemble: Unifying the Descriptions of Ligand Binding, Local
Conformational Fluctuations, and Protein Stability. Methods in Cell Biology 84,
871–891
129
Appendix A Supplementary
information for Chapter 2,
“Electrostatic Coupling in a Cluster
of Carboxylic Groups in the Active
Site of an Enzyme”
130
Table A.1. pKa values of all Asp and Glu residues in all SNase variants from this study measured at 100 mM KCl
Protein
∆+PHSc
∆+PHS/D19N
Residue
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
pKaa
2.12 ± 0.05d,e
6.54 ± 0.01e
3.83 ± 0.05e
≤ 1.7
2.16 ± 0.04d
3.81 ± 0.06
3.86 ± 0.03
2.83 ± 0.05d,e
4.32 ± 0.03
3.93 ± 0.05
3.49 ± 0.05
3.76 ± 0.04
3.31 ± 0.01
3.30 ± 0.02e
3.81 ± 0.06
3.89 ± 0.05
3.75 ± 0.06
3.75 ± 0.05
4.49 ± 0.02
5.75 ± 0.02e
3.80 ± 0.03
≤ 1.7
-
131
∆pKab
-0.79 ± 0.02
-0.03 ± 0.06
-
na
0.81 ± 0.01d,e
1.03 ± 0.02e
0.65 ± 0.01e
0.87 ± 0.01d
0.77 ± 0.03
0.76 ± 0.01
0.94 ± 0.01d,e
0.69 ± 0.01
0.65 ± 0.02
0.83 ± 0.02
0.99 ± 0.02
0.92 ± 0.01
0.88 ± 0.03e
0.82 ± 0.01
0.78 ± 0.02
0.66 ± 0.01
0.82 ± 0.01
0.85 ± 0.01
0.94 ± 0.02e
0.56 ± 0.02
-
∆+PHS/D21N
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
2.22 ± 0.005d
3.77 ± 0.01
3.86 ± 0.01
2.87 ± 0.01d,e
3.79 ± 0.01
3.85 ± 0.02
3.49 ± 0.01
3.73 ± 0.01
3.24 ± 0.01
3.19 ± 0.05e
3.83 ± 0.01
3.92 ± 0.02
3.79 ± 0.01
3.77 ± 0.01
4.50 ± 0.01
2.60 ± 0.01d
3.94 ± 0.01
≤ 1.6
2.26 ± 0.01d
3.93 ± 0.01
3.95 ± 0.01
2.93 ± 0.02d,e
4.46 ± 0.02
4.10 ± 0.03
3.63 ± 0.01
3.85 ± 0.01
132
0.06 ± 0.04
-0.04 ± 0.06
0.00 ± 0.03
0.04 ± 0.05
-0.53 ± 0.03
-0.08 ± 0.05
0.00 ± 0.05
-0.03 ± 0.04
-0.07 ± 0.01
-0.11 ± 0.05
0.02 ± 0.06
0.03 ± 0.05
0.04 ± 0.06
0.02 ± 0.05
0.01 ± 0.02
0.48 ± 0.05
0.11 ± 0.05
0.10 ± 0.04
0.12 ± 0.06
0.09 ± 0.03
0.10 ± 0.05
0.14 ± 0.04
0.17 ± 0.06
0.14 ± 0.05
0.09 ± 0.04
0.88 ± 0.01d
0.76 ± 0.01
0.76 ± 0.01
0.98 ± 0.01d,e
0.68 ± 0.01
0.69 ± 0.01
0.86 ± 0.01
0.98 ± 0.01
0.93 ± 0.01
0.85 ± 0.05e
0.84 ± 0.01
0.81 ± 0.03
0.68 ± 0.01
0.83 ± 0.01
0.83 ± 0.02
0.82 ± 0.02d
0.68 ± 0.01
0.91 ± 0.02d
0.83 ± 0.02
0.77 ± 0.02
0.96 ± 0.03d,e
0.67 ± 0.02
0.66 ± 0.02
0.88 ± 0.01
0.98 ± 0.02
∆+PHS/D40N
∆+PHS/E43Q
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
3.31 ± 0.04
3.28 ± 0.02e,f
3.93 ± 0.01
3.98 ± 0.02
3.89 ± 0.02
3.84 ± 0.02
4.56 ± 0.02
2.19 ± 0.01d,e
6.18 ± 0.01e
≤ 1.7
2.21 ± 0.005d
3.74 ± 0.01
3.75 ± 0.01
2.88 ± 0.01d,e
4.11 ± 0.01
3.77 ± 0.02
3.46 ± 0.01
3.71 ± 0.005
3.22 ± 0.01
3.22 ± 0.02e,f
3.80 ± 0.01
3.89 ± 0.02
3.67 ± 0.01
3.73 ± 0.01
4.40 ± 0.01
2.34 ± 0.01d,e
133
0.00 ± 0.04
-0.02 ± 0.03
0.12 ± 0.06
0.09 ± 0.05
0.14 ± 0.06
0.09 ± 0.05
0.07 ± 0.03
0.07 ± 0.05
-0.36 ± 0.01
0.05 ± 0.04
-0.07 ± 0.06
-0.11 ± 0.03
0.05 ± 0.05
-0.21 ± 0.03
-0.16 ± 0.04
-0.03 ± 0.05
-0.05 ± 0.04
-0.09 ± 0.01
-0.08 ± 0.03
-0.01 ± 0.06
0.00 ± 0.05
-0.08 ± 0.01
-0.02 ± 0.05
-0.09 ± 0.02
0.22 ± 0.05
0.90 ± 0.04
0.84 ± 0.03e,f
0.85 ± 0.02
0.86 ± 0.03
0.73 ± 0.02
0.86 ± 0.02
0.81 ± 0.02
0.93 ± 0.02d,e
0.97 ± 0.01e
0.90 ± 0.01d
0.80 ± 0.01
0.76 ± 0.01
1.01 ± 0.01d,e
0.73 ± 0.01
0.75 ± 0.02
0.86 ± 0.01
1.00 ± 0.01
0.94 ± 0.01
0.89 ± 0.04e,f
0.87 ± 0.01
0.86 ± 0.02
0.74 ± 0.01
0.87 ± 0.01
0.83 ± 0.01
0.81 ± 0.03d,e
∆+PHS/D19N/D40N/E43Q
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
6.16 ± 0.01e
3.69 ± 0.01e
≤ 1.7
2.21 ± 0.01d
3.76 ± 0.02
3.77 ± 0.01
2.88 ± 0.01d,e
3.65 ± 0.01
3.47 ± 0.01
3.70 ± 0.01
3.23 ± 0.01
3.27 ± 0.03e
3.80 ± 0.01
3.86 ± 0.03
3.73 ± 0.01
3.71 ± 0.01
4.42 ± 0.01
4.57 ± 0.01
≤ 1.7
2.21 ± 0.01d
3.75 ± 0.01
3.76 ± 0.01
2.90 ± 0.02d,e
134
-0.38 ± 0.01
-0.14 ± 0.01
0.05 ± 0.04
-0.05 ± 0.06
-0.09 ± 0.03
0.05 ± 0.05
-0.28 ± 0.05
-0.02 ± 0.05
-0.06 ± 0.04
-0.08 ± 0.01
-0.03 ± 0.04
-0.01 ± 0.06
-0.03 ± 0.06
-0.02 ± 0.06
-0.04 ± 0.05
-0.07 ± 0.02
-1.97 ± 0.01
-0.05 ± 0.04
-0.06 ± 0.06
-0.10 ± 0.03
0.07 ± 0.05
0.93 ± 0.01e
0.83 ± 0.01e
0.91 ± 0.01d
0.79 ± 0.02
0.75 ± 0.01
1.01 ± 0.02d,e
0.75 ± 0.01
0.89 ± 0.01
1.03 ± 0.01
0.95 ± 0.02
0.99 ± 0.04e
0.87 ± 0.01
0.82 ± 0.03
0.69 ± 0.01
0.87 ± 0.01
0.81 ± 0.01
0.93 ± 0.02
0.90 ± 0.01d
0.83 ± 0.01
0.79 ± 0.01
1.01 ± 0.03d,e
∆+PHS/R35Q
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
3.55 ± 0.01
3.49 ± 0.01
3.70 ± 0.01
3.24 ± 0.01
3.29 ± 0.01e,f
3.83 ± 0.01
3.91 ± 0.02
3.75 ± 0.02
3.74 ± 0.01
4.40 ± 0.01
3.06 ± 0.01d,e
6.05 ± 0.01e
4.27 ± 0.01
≤ 1.9
2.28 ± 0.01d
3.74 ± 0.01
3.78 ± 0.01
2.94 ± 0.01d,e
4.45 ± 0.02
3.89 ± 0.02
3.50 ± 0.02
3.73 ± 0.01
3.26 ± 0.02
3.31 ± 0.01e,f
3.87 ± 0.01
3.95 ± 0.03
135
-0.38 ± 0.05
0.00 ± 0.05
-0.06 ± 0.04
-0.07 ± 0.01
-0.01 ± 0.02
0.02 ± 0.06
0.02 ± 0.05
0.00 ± 0.06
-0.01 ± 0.05
-0.09 ± 0.02
0.94 ± 0.05
-0.49 ± 0.01
0.44 ± 0.05
0.12 ± 0.04
-0.07 ± 0.06
-0.08 ± 0.03
0.11 ± 0.03
0.13 ± 0.04
-0.04 ± 0.05
0.01 ± 0.05
-0.03 ± 0.04
-0.05 ± 0.02
0.01 ± 0.02
0.06 ± 0.06
0.06 ± 0.06
0.87 ± 0.02
0.89 ± 0.02
0.99 ± 0.01
0.94 ± 0.02
0.99 ± 0.03e,f
0.86 ± 0.01
0.85 ± 0.03
0.79 ± 0.02
0.87 ± 0.02
0.84 ± 0.01
0.88 ± 0.02d,e
0.89 ± 0.02e
0.64 ± 0.01
0.91 ± 0.01d
0.77 ± 0.01
0.75 ± 0.01
1.03 ± 0.01d,e
0.63 ± 0.02
0.61 ± 0.01
0.87 ± 0.02
0.99 ± 0.01
0.95 ± 0.02
0.96 ± 0.02e,f
0.85 ± 0.01
0.81 ± 0.03
∆+PHS/D19N/R35Q/D40N/E43Q
∆+PHS/D21N/R35Q/D40N/E43Q
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
3.77 ± 0.01
3.75 ± 0.01
4.44 ± 0.01
4.65 ± 0.01
≤ 1.9
2.22 ± 0.01d
3.85 ± 0.01
3.85 ± 0.02
2.93 ± 0.02d,e
3.67 ± 0.01
3.55 ± 0.01
3.75 ± 0.01
3.19 ± 0.03
3.29 ± 0.01e,f
3.86 ± 0.02
3.99 ± 0.03
3.81 ± 0.03
3.78 ± 0.01
4.44 ± 0.02
3.46 ± 0.03
≤ 1.9
-
136
0.02 ± 0.06
0.00 ± 0.05
-0.05 ± 0.02
-1.89 ± 0.01
0.06 ± 0.04
0.04 ± 0.06
-0.01 ± 0.04
0.10 ± 0.05
-0.26 ± 0.05
0.06 ± 0.05
-0.01 ± 0.04
-0.12 ± 0.03
-0.01 ± 0.02
0.05 ± 0.06
0.10 ± 0.06
0.06 ± 0.06
0.03 ± 0.05
-0.05 ± 0.03
1.34 ± 0.06
-
0.67 ± 0.01
0.84 ± 0.01
0.81 ± 0.02
0.90 ± 0.02
0.88 ± 0.01d
0.87 ± 0.01
0.81 ± 0.02
1.02 ± 0.03d,e
0.85 ± 0.02
0.89 ± 0.01
1.03 ± 0.02
0.88 ± 0.02
0.97 ± 0.03e,f
0.87 ± 0.02
0.89 ± 0.05
0.84 ± 0.04
0.90 ± 0.01
0.91 ± 0.03
0.96 ± 0.04
-
∆+PHS/R35Q/D40N/E43Q
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
2.27 ± 0.01d
3.80 ± 0.02
3.81 ± 0.02
2.98 ± 0.01d,e
3.76 ± 0.02
3.55 ± 0.02
3.78 ± 0.01
3.33 ± 0.03d
3.25 ± 0.03e,f
3.90 ± 0.02
4.01 ± 0.02
3.77 ± 0.02
3.78 ± 0.04
4.46 ± 0.02
3.10 ± 0.05e
5.70 ± 0.01
≤ 1.90
2.31 ± 0.01d
3.82 ± 0.01
3.86 ± 0.01
2.98 ± 0.01d,e
3.78 ± 0.01
3.56 ± 0.01
3.81 ± 0.01
137
0.11 ± 0.04
-0.01 ± 0.06
-0.05 ± 0.04
0.15 ± 0.05
-0.17 ± 0.05
0.06 ± 0.05
0.02 ± 0.04
0.02 ± 0.03
-0.05 ± 0.04
0.09 ± 0.06
0.12 ± 0.05
0.02 ± 0.06
0.03 ± 0.06
-0.03 ± 0.03
0.98 ± 0.07
-0.84 ± 0.01
0.15 ± 0.04
0.01 ± 0.06
0.00 ± 0.03
0.15 ± 0.05
-0.15 ± 0.05
0.07 ± 0.05
0.05 ± 0.04
0.89 ± 0.02d
0.82 ± 0.02
0.77 ± 0.02
1.04 ± 0.03d,e
0.86 ± 0.02
0.87 ± 0.02
0.99 ± 0.02
1.18 ± 0.09d
0.90 ± 0.08e,f
0.87 ± 0.02
0.87 ± 0.03
0.79 ± 0.02
0.88 ± 0.05
0.86 ± 0.03
0.87 ± 0.08e
0.99 ± 0.02
0.89 ± 0.02d
0.83 ± 0.01
0.80 ± 0.01
1.00 ± 0.02d,e
0.91 ± 0.01
0.89 ± 0.01
1.05 ± 0.01
PHS
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
3.32 ± 0.03
3.37 ± 0.02e,f
3.94 ± 0.01
4.00 ± 0.02
3.79 ± 0.01
3.82 ± 0.01
4.46 ± 0.01
2.05 ± 0.05d,e
6.12 ± 0.05
3.73 ± 0.02e
≤ 1.8
2.16 ± 0.03d
3.70 ± 0.01
3.68 ± 0.01
2.90 ± 0.01d,e
3.74 ± 0.03e
3.90 ± 0.02
3.76 ± 0.01
3.34 ± 0.02
3.33 ± 0.01e,f
3.91 ± 0.01
3.91 ± 0.02
3.76 ± 0.01
3.74 ± 0.01
4.16 ± 0.02
138
0.01 ± 0.03
0.07 ± 0.03
0.13 ± 0.06
0.11 ± 0.05
0.04 ± 0.06
0.07 ± 0.05
-0.03 ± 0.02
-0.07 ± 0.07
-0.42 ± 0.05
-0.10 ± 0.05
0.00 ± 0.05
-0.11 ± 0.06
-0.18 ± 0.03
0.07 ± 0.05
-0.58 ± 0.04
-0.03 ± 0.05
0.00 ± 0.04
0.03 ± 0.02
0.03 ± 0.02
0.10 ± 0.06
0.02 ± 0.05
0.01 ± 0.06
-0.01 ± 0.05
-0.33 ± 0.03
0.94 ± 0.03
0.91 ± 0.03e,f
0.92 ± 0.01
0.90 ± 0.03
0.78 ± 0.01
0.89 ± 0.01
0.85 ± 0.01
0.63 ± 0.06d,e
0.81 ± 0.06
0.84 ± 0.03e
0.81 ± 0.03d
0.81 ± 0.01
0.77 ± 0.01
0.88 ± 0.02d,e
0.76 ± 0.07e
0.79 ± 0.02
1.05 ± 0.02
1.00 ± 0.03
0.93 ± 0.03e,f
0.91 ± 0.02
0.83 ± 0.02
0.76 ± 0.01
0.87 ± 0.01
0.75 ± 0.02
pKa values and Hill coefficients obtained by fitting the modified Hill equation (Equation (2.2)) to the pH-dependence of the Cγ/Cδ chemical shift, unless
otherwise indicated. Titrations were performed at 298 K and 100 mM KCl. Values reported are those from a single titration experiment with
corresponding errors of fit, unless otherwise indicated.
b Change in pKa relative to ∆+PHS at 100 mM KCl: ∆pKa = pKavariant – pKa∆+PHS
c pKa values obtained using the data from Castañeda et al.l1 Except for Glu-73 and Glu-75, reported values are means and standard errors over 3
independent titration experiments. Reasons for discrepancies between that paper and the data presented here are explained in Chapter 2, Materials and
methods.
d pKa and Hill coefficient determined by fixing the amplitude (∆δ) of the transition to the ∆δ obtained from the fit for the same residue in ∆+PHS at 1 M
KCl
e pKa and Hill coefficient obtained by fitting a two-site model (Equation (2.2)) to the pH-dependence of the Cγ/Cδ chemical shift. Only the values
corresponding to the larger of the two transitions are reported.
f pKa and Hill coefficient determined by fixing the amplitude (∆) of the transition to the ∆ obtained from the fit for the same residue in ∆+PHS at 0.1 M
KCl
a
139
Table A.2. pKa values for all carboxylic groups in ∆+PHS and
∆+PHS/D19N/D40N/E43Q measured at 1 M KCl
Protein
Residue
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
∆+PHSc
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
∆+PHS/D19N/D40N/E43Q
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
pKaa
2.88 ± 0.02d
6.02 ± 0.01d
4.28 ± 0.01
≤ 2.1
2.71 ± 0.02
3.94 ± 0.01
3.93 ± 0.01
3.43 ± 0.01d
4.40 ± 0.01
4.08 ± 0.02
3.90 ± 0.01
4.16 ± 0.003
3.80 ± 0.01
4.04 ± 0.01d,e
4.41 ± 0.01
4.28 ± 0.04d
4.32 ± 0.01
4.08 ± 0.01
4.45 ± 0.01
5.01 ± 0.01
2.64 ± 0.01
3.87 ± 0.01
3.89 ± 0.01
3.36 ± 0.01
3.91 ± 0.01
3.87 ± 0.01
4.120 ± 0.004
3.73 ± 0.01
4.10 ± 0.12
4.40 ± 0.01
4.28 ± 0.02
4.26 ± 0.02
4.06 ± 0.01
4.49 ± 0.01
140
∆pKab
0.76 ± 0.05
-0.52 ± 0.01
0.45 ± 0.05
0.55 ± 0.04
0.13 ± 0.06
0.07 ± 0.03
0.60 ± 0.05
0.08 ± 0.03
0.15 ± 0.05
0.41 ± 0.05
0.4 ± 0.04
0.49 ± 0.01
0.74 ± 0.02
0.60 ± 0.06
0.39 ± 0.06
0.57 ± 0.06
0.33 ± 0.05
-0.04 ± 0.02
0.44 ± 0.01
0.43 ± 0.01
0.12 ± 0.01
0.13 ± 0.01
0.46 ± 0.02
0.36 ± 0.01
0.38 ± 0.01
0.42 ± 0.01
0.49 ± 0.01
0.81 ± 0.12
0.57 ± 0.01
0.37 ± 0.03
0.51 ± 0.03
0.32 ± 0.01
0.09 ± 0.01
na
0.83 ± 0.04d
0.94 ± 0.02d
0.81 ± 0.01
0.90 ± 0.03
0.96 ± 0.01
0.92 ± 0.01
1.01 ± 0.02d
0.81 ± 0.02
0.84 ± 0.02
0.98 ± 0.02
1.03 ± 0.01
0.91 ± 0.02
1.00 ± 0.02d,e
0.89 ± 0.03
0.76 ± 0.04d
0.83 ± 0.01
0.95 ± 0.01
0.88 ± 0.01
0.95 ± 0.02
0.93 ± 0.01
0.90 ± 0.01
0.88 ± 0.01
0.95 ± 0.02
0.94 ± 0.02
0.97 ± 0.02
1.00 ± 0.01
0.88 ± 0.02
1.1 ± 0.2
0.90 ± 0.01
0.80 ± 0.02
0.83 ± 0.02
0.90 ± 0.01
0.94 ± 0.01
pKa values and Hill coefficients obtained by fitting the modified Hill equation (Equation (2.2)) to the
pH-dependence of the Cγ/Cδ chemical shift, unless otherwise indicated. Titrations were performed
at 298 K and 1 M KCl. Values reported are those from a single titration experiment with
corresponding errors of fit.
b Change in pKa relative to the same variant at 100 mM KCl: ∆pKa = pKa1M – pKa100mM
c pKa values obtained using the data from Castañeda et al.l1 Reasons for discrepancies between that
paper and the data presented here are explained in Materials and Methods.
d pKa and Hill coefficient obtained by fitting a two-site model (Equation (2.3)) to the pH-dependence
of the Cγ/Cδ chemical shift. Only the values corresponding to the larger of the two transitions are
reported.
e pKa and Hill coefficient determined by fixing the amplitude (∆) of the transition to the ∆ obtained
from the fit for the same residue in ∆+PHS at 0.1 M KCl
a
141
Table A.3. X-Ray data collection and refinement statistics for ∆+PHS/D21N
PDB accession code
Data collection
Space group
Cell dimensions
a (Å)
b (Å)
c (Å)
β (°)
Wavelength (Å)
Temperature (K)
Resolutiona (Å)
Rmergea,b (%)
I/σ(I)a
Redundancya
No. unique reflectionsa
Completenessa (%)
Wilson B-factor (Å2)
Refinement
Resolutiona (Å)
No. reflectionsa
Rworka,c (%)
Rfreea,c (%)
No. molecules per asymmetric unit
No. atoms
Protein
Ligand
Water
Average B-factors (Å2)
Protein atoms
Ligand
Water
R.M.S.D.
Bond lengths (Å)
Bond angles (°)
Ramachandran plot
No. in most favored regions (%)
No. in additionally allowed regions (%)
No. in generously allowed regions (%)
No. in disallowed regions (%)
Total No. Non-Gly, Non-Pro Residues
a The
3LX0
P21
30.75
60.38
34.53
97.76
0.9795
100
27.2–1.45 (1.48-1.45)
6.7 (30.0)
16.4 (6.9)
6.7 (5.8)
21,820 (1013)
98.3 (93.4)
24.0
24.45–1.50 (1.54-1.50)
19,778 (1307)
17.9 (21.9)
22.2 (25.2)
1
1140
30
142
17.8
18.8
27.6
0.02
1.9
105 (86.8)
15 (12.4)
0 (0)
1 (0.8)
121
value in parentheses is for the highest resolution shell
142
b
Rmerge Ihkl, j Ihkl
hkl
I
j
hkl
hkl, j
where Ihkl ,j represents the jth observation of the intensity
j
of a unique set of indices hkl, and <Ihkl> is the mean intensity for this set of indices
c
Rwork Fobs Fcalc
hkl
F
obs
calculated using 95% of reflections, while Rfree reports the same
hkl
calculation using the remaining 5% of reflections. Rfree calculated using the same set of reflections
used to calculate Rfree for the molecular replacement model.
143
Figure A.1. (following page) (a) Overlay of the structures of ∆+PHS (PDB accession
code 3BDC1, white) and ∆+PHS/D21N (PDB ID 3LX0, green), showing the ionizable
groups in the active site. (b) Same for NVIAGA/E75A (PDB ID 2RDF2, white) and
∆+PHS/D21N (green).
144
145
A.1 References
1. Castañeda, C.A., Fitch, C.A., Majumdar, A., Khangulov, V., Schlessman, J.L. &
García‐Moreno, B.E. (2009). Molecular determinants of the pKa values of Asp
and Glu residues in staphylococcal nuclease. Proteins: Structure, Function, and
Bioinformatics 77, 570–588
2. Baran, K.L., Chimenti, M.S., Schlessman, J.L., Fitch, C.A., Herbst, K.J. & GarcíaMoreno, B. (2008). Electrostatic effects in a network of polar and ionizable
groups in staphylococcal nuclease. Journal of Molecular Biology 379, 1045–1062
146
Appendix B Supplementary
information for Chapter 3,
“Conformational Reorganization of
the Backbone Influences the pKa
Values of Ionizable Groups in
Proteins”
147
Table B.1: pKa values of select Asp & Glu residues measured by NMR spectroscopy.a
Protein
Residue
pKab
∆pKac
Asp-19
2.12 ± 0.05f,g
Asp-21
6.54 ± 0.01g
g
Asp-40
3.83 ± 0.05
Asp-77
≤ 1.7e
Asp-83
Asp-95
2.16 ± 0.04f
Asp-143
3.81 ± 0.06
Asp-146
3.86 ± 0.03
f,g
Glu-10
2.83 ± 0.05
Glu-43
4.32 ± 0.03
∆+PHSd
Glu-52
3.93 ± 0.05
Glu-57
3.49 ± 0.05
Glu-67
3.76 ± 0.04
Glu-73
3.31 ± 0.01
Glu-75
3.30 ± 0.02g
Glu-101
3.81 ± 0.06
Glu-122
3.89 ± 0.05
Glu-129
3.75 ± 0.06
Glu-135
3.75 ± 0.05
Glu-142
4.49 ± 0.02
Asp-19
2.18 ± 0.01f,g 0.06 ± 0.05
Asp-21
6.52 ± 0.05g -0.02 ± 0.05
Asp-40
3.79 ± 0.02g -0.04 ± 0.05
Asp-77
≤ 1.6e
Asp-83
f
Asp-95
2.15 ± 0.01
-0.01 ± 0.04
Asp-143
3.75 ± 0.01
-0.06 ± 0.06
Asp-146
3.76 ± 0.01
-0.10 ± 0.03
Glu-10
2.90 ± 0.01f,g 0.07 ± 0.05
Glu-43
4.23 ± 0.01
-0.09 ± 0.03
∆+PHS/P11G
Glu-52
3.85 ± 0.02
-0.08 ± 0.05
Glu-57
3.45 ± 0.01
-0.04 ± 0.05
Glu-67
3.71 ± 0.01
-0.05 ± 0.04
Glu-73
3.20 ± 0.02
-0.11 ± 0.02
g
Glu-75
3.17 ± 0.05
-0.13 ± 0.05
Glu-101
3.84 ± 0.01
0.03 ± 0.06
Glu-122
3.89 ± 0.02
0.00 ± 0.05
Glu-129
3.73 ± 0.01
-0.02 ± 0.06
Glu-135
3.73 ± 0.01
-0.02 ± 0.05
Glu-142
4.40 ± 0.01
-0.09 ± 0.02
f,g
Asp-19
2.31 ± 0.03
0.19 ± 0.06
∆+PHS/A60G
Asp-21
6.59 ± 0.01g
0.05 ± 0.01
g
Asp-40
3.94 ± 0.01
0.11 ± 0.05
148
∆+PHS/A69G
∆+PHS/M98G
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
≤ 1.7e
2.38 ± 0.01f
3.87 ± 0.01
3.86 ± 0.01
3.02 ± 0.01f,g
4.35 ± 0.01
3.94 ± 0.02
3.67 ± 0.01
3.85 ± 0.01
3.38 ± 0.02
3.45 ± 0.01f,g
3.98 ± 0.01
4.01 ± 0.02
3.92 ± 0.01
3.86 ± 0.01
4.48 ± 0.01
2.18 ± 0.03f,g
6.52 ± 0.03g
3.79 ± 0.04g
≤ 1.6e
2.77 ± 0.02f
3.82 ± 0.02
3.86 ± 0.02
2.86 ± 0.01f,g
4.33 ± 0.02
3.98 ± 0.04
3.52 ± 0.02
3.79 ± 0.02
3.20 ± 0.05
3.27 ± 0.04g
3.77 ± 0.04
3.80 ± 0.05
3.66 ± 0.04
3.75 ± 0.02
4.51 ± 0.01
2.38 ± 0.08f,g
6.54 ± 0.02g
3.76 ± 0.07g
≤ 2.5e
2.25 ± 0.05f
3.74 ± 0.02
3.74 ± 0.01
149
0.22 ± 0.04
0.06 ± 0.06
0.00 ± 0.03
0.19 ± 0.05
0.03 ± 0.03
0.01 ± 0.05
0.18 ± 0.05
0.09 ± 0.04
0.07 ± 0.02
0.15 ± 0.02
0.17 ± 0.06
0.12 ± 0.05
0.17 ± 0.06
0.11 ± 0.05
-0.01 ± 0.02
0.06 ± 0.06
-0.02 ± 0.03
-0.04 ± 0.06
0.61 ± 0.04
0.01 ± 0.06
0.00 ± 0.04
0.03 ± 0.05
0.01 ± 0.04
0.05 ± 0.06
0.03 ± 0.05
0.03 ± 0.04
-0.11 ± 0.05
-0.03 ± 0.04
-0.04 ± 0.07
-0.09 ± 0.07
-0.09 ± 0.07
0.00 ± 0.05
0.02 ± 0.02
0.26 ± 0.09
0.00 ± 0.02
-0.07 ± 0.09
0.09 ± 0.06
-0.07 ± 0.06
-0.12 ± 0.03
∆+PHS/A130G
∆+PHS/M98A
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
Glu-52
Glu-57
Glu-67
2.94 ± 0.02f,g
4.24 + 0.02
3.80 ± 0.05
3.46 ± 0.07
3.73 ± 0.02
3.24 ± 0.01f
3.92 ± 0.05
3.32 ± 0.01f
3.86 ± 0.06
3.66 ± 0.08
3.83 ± 0.03
4.37 ± 0.01
2.37 ± 0.01f,g
6.56 ± 0.01g
3.96 ± 0.01g
≤ 1.6e
2.37 ± 0.01f
3.87 ± 0.01
3.85 ± 0.01
3.04 ± 0.01f,g
4.36 ± 0.01
3.96 ± 0.01
3.61 ± 0.01
3.85 ± 0.01
3.39 ± 0.02
3.50 ± 0.03g
4.02 ± 0.01
4.05 ± 0.02
3.86 ± 0.02
3.83 ± 0.02
4.49 ± 0.01
2.32 ± 0.07f,g
6.55 ± 0.02g
3.71 ± 0.08g
≤ 2.7e
2.13 ± 0.06f
3.77 ± 0.02
3.80 ± 0.03
2.81 ± 0.01f,g
4.31 ± 0.03
3.94 ± 0.03
3.43 ± 0.05
3.68 ± 0.04
150
0.11 ± 0.05
-0.08 ± 0.04
-0.13 ± 0.07
-0.03 ± 0.09
-0.03 ± 0.04
-0.07 ± 0.01
0.62 ± 0.05
-0.49 ± 0.06
-0.03 ± 0.08
-0.09 ± 0.10
0.08 ± 0.06
-0.12 ± 0.02
0.25 ± 0.05
0.02 ± 0.01
0.13 ± 0.05
0.21 ± 0.04
0.06 ± 0.06
-0.01 ± 0.03
0.21 ± 0.05
0.04 ± 0.03
0.03 ± 0.05
0.12 ± 0.05
0.09 ± 0.04
0.08 ± 0.02
0.20 ± 0.04
0.21 ± 0.06
0.16 ± 0.05
0.11 ± 0.06
0.08 ± 0.05
0.00 ± 0.02
0.2 ± 0.09
0.01 ± 0.02
-0.12 ± 0.09
-0.03 ± 0.07
-0.04 ± 0.06
-0.06 ± 0.04
-0.02 ± 0.05
-0.01 ± 0.04
0.01 ± 0.06
-0.06 ± 0.07
-0.08 ± 0.06
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
Glu-43
∆+PHS/A58G/A60G
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
Glu-135
Glu-142
Asp-19
Asp-21
Asp-40
Asp-77
Asp-83
Asp-95
Asp-143
Asp-146
Glu-10
∆+PHS/A128G/A130G
Glu-43
Glu-52
Glu-57
Glu-67
Glu-73
Glu-75
Glu-101
Glu-122
Glu-129
3.11 ± 0.01f
3.91 ± 0.16f
3.27 ± 0.02f
3.93 ± 0.06
3.75 ± 0.10
3.86 ± 0.03
4.43 ± 0.01
2.18 ± 0.11f,g
6.33 ± 0.01g
3.88 ± 0.05g
≤ 1.8e
1.93 ± 0.05f
3.92 ± 0.03
3.99 ± 0.03
2.80 ± 0.01f,g
4.36 ± 0.01
4.02 ± 0.01f
3.61 ± 0.01f
3.78 ± 0.02
3.20 ± 0.01f
3.19 ± 0.01f,g
3.83 ± 0.05
3.73 ± 0.08
3.84 ± 0.05
3.81 ± 0.03
4.48 ± 0.01
2.22 ± 0.08f,g
6.62 ± 0.02g
3.93 ± 0.03g
≤ 1.8e
2.13 ± 0.04f
3.93 ± 0.03
4.00 ± 0.02
2.88 ± 0.01f,g
4.36 ± 0.01
4.09 ± 0.01
3.67 ± 0.02
3.84 ± 0.02
3.28 ± 0.01f
3.31 ± 0.02f,g
3.97 ± 0.02h
3.89 ± 0.07
3.69 ± 0.05
151
-0.20 ± 0.01
0.60 ± 0.16
-0.54 ± 0.06
0.04 ± 0.07
0.00 ± 0.12
0.11 ± 0.06
-0.06 ± 0.02
0.06 ± 0.12
-0.21 ± 0.01
0.05 ± 0.07
-0.23 ±0.06
-0.11 ± 0.07
0.13 ± 0.04
-0.03 ± 0.05
0.04 ± 0.03
0.09 ± 0.05
0.12 ± 0.05
0.02 ± 0.04
-0.11 ± 0.01
-0.11 ± 0.02
0.02 ± 0.08
-0.16 ± 0.09
0.09 ± 0.08
0.06 ± 0.06
-0.01 ± 0.02
0.10 ± 0.09
0.08 ± 0.02
0.10 ± 0.06
-0.03 ± 0.06
0.12 ± 0.07
0.14 ± 0.04
0.05 ± 0.05
0.04 ± 0.03
0.16 ± 0.05
0.18 ± 0.05
0.08 ± 0.04
-0.03 ± 0.01
0.00 ± 0.03
0.16 ± 0.06
0.00 ± 0.09
-0.06 ± 0.08
Glu-135
4.08 ± 0.02
0.33 ± 0.05
Glu-142
4.52 ± 0.01
0.03 ± 0.02
a Measurements were performed at 298 K and 100 mM KCl.
b pKa values were obtained by fitting a single-site modified Hill equation to the data,
unless otherwise indicated. Values reported are from a single titration experiment
with corresponding goodness of fit, unless otherwise indicated.
c Change in pKa relative to ∆+PHS
d pKa values for ∆+PHS are means & standard errors over 3 independent titration
experiments, using the data from Castañeda et al1.
e Upper limit for Asp-77 pKa obtained by fitting the data to a two-site modified Hill
equation with a fixed Hill coefficient of 1 and a fixed ∆δ of 1.85 ppm for the low-pH
transition
f Fit performed by fixing the amplitude of the titration (∆δ) to the value obtained
from the titration of the same residue in ∆+PHS at 1M KCl1 or at 100 mM (for Glu-73
& Glu-75)
g pKa values obtained by fitting a two-site modified Hill equation to the data. Only
values corresponding to the larger of the two transitions are reported.
h Fit performed by fixing ∆δ to the largest value obtained for titration of other Glu
residues (4.45)
152
Table B.2: Crystallographic statistics for ∆+PHS/M98G and ∆+PHS/A69G
Variant
∆+PHS/M98G
∆+PHS/A69G
2+
Ca & pdTp present?
Yes
No
Yes
No
PDB accession code
3S9W
3SK8
3SR1
3T13
Data collection:
Space Group
P21
P41
P21
P21
Unit cell dimensions:
a (Å)
31.21
48.37
31.22
46.6
b (Å)
60.53
48.37
60.65
63.76
c (Å)
38.35
63.45
38.09
49.87
β (°)
93.13
90
93.69
91.92
Wilson B-factor
30.3
34.3
22.6
27.6
50.0-1.9
50.0-1.9
50-1.45
50-1.80
Resolution (Å)
(1.93-1.90)a
(1.93-1.9)
(1.48-1.45)
(1.83-1.80)
Completeness (%)
98.9 (90.4)
99.7 (99.3)
99.7 (99.7)
99.9 (99.3)
Rmerge (%)
5.3 (24.7)
7.3 (28.3)
4.9 (27.9)
8.5 (25.9)
I/σ(I)
14.9 (5.9)
13.3 (10.2)
17.7 (6.4)
10.5 (7.2)
Redundancy
3.9 (3.5)
13.5 (13.1)
7.1 (6.2)
7.3 (6.9)
# unique reflections
11311 (519)
11646 (581)
25258 (1230) 27161 (1331)
Refinement:
# reflections
# reflections in Rfree set
Resolution (Å)
Rwork (%)
Rfree (%)
# molecules/asymmetric unit
# atoms:
Protein
Water
11284 (816)
538 (43)
38.3-1.90
(1.94-1.90)
17.12 (21.10)
21.15 (24.40)
1
11629 (857)
555 (52)
48.37-1.90
(1.95-1.90)
16.5 (18.1)
21.0 (24.9)
1
25078 (1860)
2487 (195)
30.33-1.45
(1.49-1.45)
16.4 (22.6)
20.2 (26.1)
1
27144 (1961)
1363 (99)
49.85-1.80
(1.84-1.80)
15.6 (20.4)
19.9 (25.5)
2
1029
109
1029
96
1032
153
2073
266
153
Ligand
25
18
Ions
1
0
Average B-factors:
Protein atoms
26.7
30
Water
20.1
37
Ligand
19.6
31.7
RMSD from ideal:
Bond Lengths (Å)
0.019
0.02
Bond Angles (°)
1.642
1.704
# of TLS groups
5
7
Molprobity validation:
Rotamer outliers (%)
0
1.89
Ramachandran outliers (%)
0
0
Ramachandran favored (%)
95.2
97.64
a Values in parentheses refer to the highest-resolution shell
n
b
c
Rwork Fo hkl Fc hkl
hkl
48
1
17.5
28.3
11.8
19.6
31
26.3
0.019
1.861
7
0.018
1.623
9 (2/7)
0
0
95.28
1.86
0
97.65
n
Rmerge Ii hkl Ihkl
hkl i1
25
1
Ihkl
hkl i1
F hkl calculated using the 95% of reflections used in model building, while Rfree reports the
o
hkl
same value calculated using the remaining 5% of reflections. For A69G with Ca2+ and pdTp, Rfree was calculated using the same
set of reflections used to calculate Rfree for the molecular replacement model, otherwise the Rfree set was chosen randomly.
154
Table B.3: RMSD of Gly variant crystal structures relative to ∆+PHS
PDB ID
Cα RMSD
All-atom RMSD
3S9W
0.116
0.162
3SR1
0.123
0.165
3SK8
0.283
0.357
3T13, chain A
0.271
0.368
3T13, chain B
0.300
0.387
155
Table B.4: Hydrogen exchange rates measured in ∆+PHS and Gly variants.a
∆+PHS
∆+PHS/A69G
b
Residue
∆Gex (kcal/mol)
∆Gex (kcal/mol)b
∆∆Gexc
10
4.25 ± 0.02
4.35 ± 0.02
-0.1 ± 0.03
12
5.88 ± 0.02
5.78 ± 0.04
0.1 ± 0.04
13
5.468 ± 0.007
5.431 ± 0.005
0.038 ± 0.008
14
1.82 ± 0.03
1.94 ± 0.02
-0.12 ± 0.03
15
> 6.1d
> 5.7
16
6.73 ± 0.02
6.55 ± 0.03
0.19 ± 0.04
18
4.92 ± 0.011
4.831 ± 0.008
0.089 ± 0.013
19
5.448 ± 0.004
5.392 ± 0.007
0.056 ± 0.008
21
7.503 ± 0.006
7.362 ± 0.007
0.14 ± 0.009
22
> 8.1
> 7.7
23
7.19 ± 0.09
> 6.3
< 0.9
24
> 7.1
> 6.8
25
> 6.8
> 6.4
26
> 7.1
> 6.7
27
5.81 ± 0.008
5.75 ± 0.012
0.06 ± 0.015
30
6.347 ± 0.008
6.328 ± 0.008
0.019 ± 0.011
32
7.05 ± 0.04
7.29 ± 0.1
-0.24 ± 0.11
34
7.99 ± 0.14
>7
<1
35
> 7.6
> 7.2
36
7.32 ± 0.06
> 6.5
< 0.8
37
> 6.3
> 5.9
38
2.125 ± 0.004
2.094 ± 0.009
0.03 ± 0.01
39
> 6.1
> 5.8
40
5.241 ± 0.003
5.187 ± 0.006
0.054 ± 0.007
e
51
< 3.2
3.04 ± 0.21
< 0.2
52
3.14 ± 0.03
3.07 ± 0.02
0.07 ± 0.04
54
< 2.2
< 2.2
156
∆+PHS/M98G
∆Gex (kcal/mol)b
∆∆Gexc
3.94 ± 0.02
0.31 ± 0.03
5.312 ± 0.003
0.56 ± 0.02
5.273 ± 0.003
0.195 ± 0.007
1.83 ± 0.1
-0.01 ± 0.11
6.92 ± 0.15
> -0.8
6.216 ± 0.005
0.52 ± 0.02
4.833 ± 0.009
0.087 ± 0.014
5.367 ± 0.004
0.082 ± 0.006
7.274 ± 0.004
0.228 ± 0.007
> 8.2
> 6.8
< 0.4
> 7.2
> 6.8
> 7.2
5.844 ± 0.006
-0.035 ± 0.01
6.432 ± 0.004
-0.085 ± 0.009
7.09 ± 0.04
-0.04 ± 0.05
> 7.4
< 0.6
> 7.7
>7
< 0.3
> 6.4
2.254 ± 0.008
-0.13 ± 0.009
> 6.2
4.923 ± 0.004
0.317 ± 0.005
< 3.24
3.27 ± 0.04
-0.13 ± 0.05
< 2.29
55
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
82
83
85
86
87
88
89
6.828 ± 0.013
<2
7.05 ± 0.04
7.27 ± 0.03
7.6 ± 0.02
7.7 ± 0.1
7.82 ± 0.1
8 ± 0.07
8.05 ± 0.08
7.79 ± 0.05
6.94 ± 0.13
>7
3.108 ± 0.014
4.732 ± 0.012
< 2.4
4.386 ± 0.01
1.81 ± 0.02
> 6.9
> 6.2
>7
3.61 ± 0.009
5.146 ± 0.006
2.891 ± 0.009
3.083 ± 0.005
4.87 ± 0.02
3.38 ± 0.02
3.12 ± 0.03
5.71 ± 0.02
6.248 ± 0.007
6.22 ± 0.02
6.84 ± 0.02
< 1.9
> 6.8
7.31 ± 0.12
7.9 ± 0.09
> 6.7
>7
> 7.2
> 7.1
> 7.2
> 6.2
> 6.7
< 2.8
< 3.2
< 2.6
3.488 ± 0.006
1.69 ± 0.02
> 6.5
> 5.9
> 6.7
3.685 ± 0.006
5.159 ± 0.008
2.879 ± 0.004
3.073 ± 0.006
4.85 ± 0.02
3.363 ± 0.013
3.06 ± 0.02
5.7 ± 0.03
6.239 ± 0.009
6.41 ± 0.05
-0.01 ± 0.02
< 0.2
-0.04 ± 0.12
-0.3 ± 0.1
<1
< 0.8
< 0.8
< 0.9
< 0.6
< 0.7
> 0.3
> 1.5
0.898 ± 0.011
0.12 ± 0.03
-0.076 ± 0.01
-0.013 ± 0.01
0.012 ± 0.01
0.01 ± 0.008
0.02 ± 0.03
0.02 ± 0.02
0.07 ± 0.04
0.01 ± 0.03
0.009 ± 0.011
-0.19 ± 0.05
157
6.944 ± 0.014
< 2.01
7.43 ± 0.06
7.509 ± 0.011
7.82 ± 0.02
> 7.1
> 7.5
8.42 ± 0.07
8.73 ± 0.1
> 7.6
> 6.7
> 7.1
2.99 ± 0.03
4.658 ± 0.01
< 2.44
4.385 ± 0.008
1.756 ± 0.014
>7
> 6.3
> 7.1
< 1.96
3.493 ± 0.01
< 2.19
2.609 ± 0.012
3.658 ± 0.014
3.088 ± 0.012
3.112 ± 0.01
4.579 ± 0.015
4.999 ± 0.007
5.182 ± 0.006
-0.12 ± 0.02
-0.38 ± 0.07
-0.23 ± 0.03
-0.21 ± 0.03
< 0.5
< 0.4
-0.42 ± 0.1
-0.68 ± 0.13
< 0.2
< 0.3
0.12 ± 0.03
0.07 ± 0.02
0.001 ± 0.012
0.05 ± 0.02
> 1.7
1.654 ± 0.012
> 0.7
0.474 ± 0.013
1.21 ± 0.02
0.29 ± 0.02
0.01 ± 0.03
1.13 ± 0.02
1.249 ± 0.01
1.04 ± 0.02
90
91
92
93
94
95
97
99
100
101
102
103
104
105
106
107
108
109
110
111
112
122
125
126
127
128
129
130
131
132
7.55 ± 0.07
6.59 ± 0.11
> 6.5
7.43 ± 0.14
> 7.5
4.174 ± 0.008
6.833 ± 0.01
> 6.6
8.35 ± 0.11
> 7.7
7.44 ± 0.06
6.86 ± 0.07
6.74 ± 0.15
7.63 ± 0.06
> 7.8
>8
> 6.8
7.66 ± 0.07
> 7.3
4.447 ± 0.01
3.835 ± 0.008
4.082 ± 0.008
6.55 ± 0.05
> 7.2
5.236 ± 0.003
7 ± 0.06
> 7.2
> 7.2
5.386 ± 0.003
> 7.7
> 6.7
6.63 ± 0.12
> 6.1
> 6.3
> 7.1
3.009 ± 0.014
7.26 ± 0.03
> 6.2
> 7.5
> 7.3
> 6.8
> 6.2
> 5.8
> 6.9
> 7.4
> 7.7
> 6.5
> 6.7
>7
4.409 ± 0.011
3.77 ± 0.005
4.037 ± 0.012
> 5.9
> 6.8
5.232 ± 0.003
7.47 ± 0.12
> 6.9
> 6.8
5.351 ± 0.003
> 7.3
< 0.8
-0.05 ± 0.16
< 1.1
1.17 ± 0.02
-0.42 ± 0.03
< 0.9
< 0.6
< 0.6
<1
< 0.7
< 0.9
0.038 ± 0.015
0.065 ± 0.009
0.045 ± 0.014
< 0.6
0.003 ± 0.004
-0.47 ± 0.13
0.035 ± 0.005
158
> 7.2
6.59 ± 0.08
6.04 ± 0.05
> 6.8
> 7.5
3.877 ± 0.005
7.118 ± 0.01
> 6.8
6.388 ± 0.005
5.467 ± 0.006
> 7.3
> 6.7
> 6.2
> 7.4
> 7.9
> 8.1
> 6.9
> 7.2
> 7.4
4.495 ± 0.007
3.769 ± 0.005
4.05 ± 0.03
4.967 ± 0.008
6.6 ± 0.04
5.067 ± 0.01
6.79 ± 0.04
> 7.3
> 7.3
5.341 ± 0.004
> 7.7
< 0.4
0 ± 0.13
> 0.4
< 0.6
0.298 ± 0.01
-0.286 ± 0.014
1.96 ± 0.11
> 2.2
< 0.2
< 0.2
< 0.5
< 0.2
< 0.5
-0.048 ± 0.012
0.066 ± 0.009
0.03 ± 0.03
1.59 ± 0.05
> 0.6
0.168 ± 0.011
0.21 ± 0.07
0.045 ± 0.005
133
> 7.3
>7
> 7.4
134
7.29 ± 0.04
> 7.1
< 0.2
7.58 ± 0.03
-0.29 ± 0.05
135
5.871 ± 0.007
5.823 ± 0.007
0.049 ± 0.01
5.962 ± 0.007
-0.09 ± 0.01
136
6.26 ± 0.011
6.34 ± 0.02
-0.08 ± 0.02
6.317 ± 0.008
-0.058 ± 0.014
137
7.22 ± 0.12
> 6.4
< 0.8
> 6.8
< 0.4
138
3.301 ± 0.013
3.293 ± 0.013
0.01 ± 0.02
3.331 ± 0.014
-0.03 ± 0.02
139
7.05 ± 0.09
> 6.5
< 0.6
7.44 ± 0.11
-0.4 ± 0.15
140
> 6.5
> 6.1
> 6.6
141
6.397 ± 0.008
6.371 ± 0.005
0.026 ± 0.009
6.45 ± 0.003
-0.054 ± 0.008
a Measurements were performed at 298K, 100 mM KCl, pH* 5.05-5.15.
b Free energy of exchange (∆Gex) was calculated as RTln(kint/kex.) Values for kint were calculated based on sequence and
experimental conditions as described in Materials and Methods.
c Difference in ∆Gex between the Gly variant and ∆+PHS
d ∆Gex for residues exhibiting less than 20% exchange are given as lower limits, as described in Materials and Methods.
e ∆Gex for residues that exchange within the dead time of the experiment are given as upper limits, as described in Materials
and Methods.
159
Figure B.1: Shifts of Asp & Glu pKa in variants M98G, M98A, A58G/A60G, and
A128G/A130G relative to ∆+PHS.
B.1 References
1. Castañeda, C.A., Fitch, C.A., Majumdar, A., Khangulov, V., Schlessman, J.L. &
García‐ Moreno, B.E. (2009). Molecular determinants of the pKa values of Asp
and Glu residues in staphylococcal nuclease. Proteins: Structure, Function, and
Bioinformatics 77, 570–588
160
Vita
Brian M. Doctrow was born on October 10, 1983 in Baltimore, Maryland, where he
lived until going to college. From the time he first could read, he developed a
passion for learning and a natural curiosity about the world around him. His
interest in science became fully mature in high school, thanks in large part to the
enthusiasm of his tenth-grade biology teacher. In the fall of 2002, Brian entered
Rice University in Houston, Texas, where he majored in physics with a biophysics
concentration. He graduated with a B.S. in physics in May of 2006. Following
graduation, he spent a year as a post-baccalaureate research fellow at the National
Institute of Alcoholism and Alcohol Abuse in Rockville, Maryland. There he worked
with Drake Mitchell, studying how the properties of rhodopsin are affected by
different methods of sample preparation.
Brian chose to study biophysics to
combine his interests in the quantitative aspects of physics with the problems of
chemistry and biology.
He enrolled in the graduate Program in Molecular
Biophysics at Johns Hopkins University, matriculating in August 2007. Under the
guidance of his advisor, Bertrand García-Moreno, Brian studied electrostatic effects
in proteins from both an experimental and computational perspective.
After
receiving his Ph.D., Brian hopes to become a science journalist and writer to help
raise understanding and awareness of science in the general public.
161