Acidity, by Lisa Totten, Rutgers University
advertisement

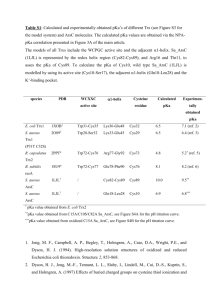
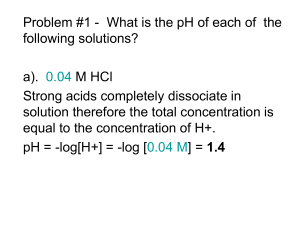
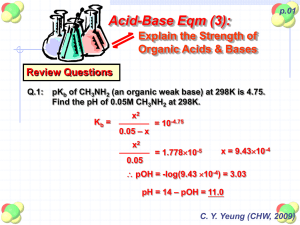
Chapter 8: Organic Acids and Bases Acid/Base Reactions • Some organic chemicals have exchangeable protons (acids) or lone pair electrons which can accept hydrogen (bases) • Ionized form of these compounds acts very differently from the neutral form (different HLC, Kow, etc) • Proton transfer reactions are usually very fast and reversible, so we can treat them as an equilibrium process Acidity Constant Organic acids: HA + H2O H3O+ + Achoosing pure water as a reference state: H+ + H2O H3O+ by convention, DG = 0, K = 1 Thus, HA H+ + A- ; lnKa = -(DG /RT) At equilibrium: ln K a ln ' H D G RT H A H ' A A o ' HA Ka = acid dissociation constant typically measure activity of H+, and conc of HA, A(mixed acidity constant) at low ionic strength, 1 ln K a ln H A HA A log log K HA a pH pH pK a when pH = pKa, [A-] = [HA] For organic bases, treatment is similar: B + H2O BH+ + OHKb ' OH OH ' B ' BH BH B written as acidity constant: BH+ B + H+ Ka H B BH ' H ' BH ' B pKa + pKb = pKw = 14 at 25C Ka * Kb = Kw = 10-14 at 25C carboxylic acids amines acids phenols bases heterocycles with N Important functional groups: Acids: CH3-OH O C H 3 -C -O H alcohols (pKa > 14) Carboxylic acids (pKa = 4.75) P henols (pK a = 9.82) Bases: NH3 ammonia (pKa = 9.25) CH3NH2 primary amine (pKa = 10.66) (CH3)2NH secondary amine (pKa = 10.73) (CH3)3N tertiary amine (pKa = 9.81) benzoic acids (pK a = 4.19) anilines (pKa = 4.63) N pyridines (pKa = 5.42) Temperature effect on pKa recall that the effect of temperature on any equilibrium constant: ln K ia T 2 K ia T 1 DrH R 0 1 1 T T 2 1 for strong acids, DrHo is very small DrHo increases as pKa increases (weaker acids have higher temperature dependence) (Why?) hmmm… what is the DrS of a proton transfer reaction? Speciation in natural waters Q: Does the presence of an organic acid affect the pH of the water? A: Probably not. Why? Natural waters are usually buffered by carbonate (among other things). If carbonate is present at 10-3 M and the pH is neutral, then addition of acid at 10-5 M (a factor of 100 less than the buffer) will have virtually no effect on pH. Speciation in natural waters fraction of acid in the neutral form: HA 1 HA A 1 [ A ] 1 1 10 [ HA ] fraction of base in the neutral form: 1 ( pH pK a ) Chemical structure and pKa We are mostly concerned with compounds for which 3 < pKa <11 aliphatic and aromatic carboxyl groups aromatic hydroxyl groups (phenols) aliphatic and aromatic amino groups N heterocycles aliphatic or aromatic thiols These classes of compounds have pKa’s which vary widely Why? Substituents can have a dramatic effect on the pKa of the compound Substituent effects are of three types: Inductive effects positive (electron donating) for O-, NH-, alkyl negative (electron withdrawing) for NO2, halogen, ether, phenyl, etc. Delocalization effects (resonance) positive for halogen, NH2, OH, OR negative for NO2, others Proximity effects intramolecular hydrogen bonding steric effects Inductive effects pKa acetic acid 4.75 propanoic acid 4.87 butyric acid 4.85 4-chlorobutyric acid 4.52 3-chlorobutyric acid 4.05 2-chlorobutyric acid 2.86 proximity is crucial alkyl groups are weakly electron donating chlorines are strongly electron withdrawing Delocalization effects (resonance) positive for halogen, NH2, OH, OR negative for NO2, others Example: chlorinated phenols: phenol 2-chlorophenol 3-chlorophenol 4-chlorophenol 2.4-dichlorophenol 2,4,5-trichlorophenol 2,4,6-trichlorophenol 2,3,4,5-tetrachlorophenol 2,3,4,6-tetrachlorophenol pentachlorophenol 9.92 8.44 8.98 9.29 7.85 6.91 6.19 6.35 5.40 4.83 general reduction in pKa due to chlorine substitution is caused by inductive (electron withdrawing effect) specific reduction in pKa (dependent on chlorine position) is caused by resonance effect Resonance effect of hydroxyl and amino groups Resonance effects are heavily influenced by position Proximity effects highly specific interactions due to proximity of substituents to the functional group: often difficult to quantify intramolecular hydrogen bonding steric effects examples Predicting acidity constant For some specific aromatic structures, acidity constant can be estimated via: Hammett Correlation effects of substituents are quantified via values pK a pK aH i i the pKa of the unknown = the pKa of the unsubstituted parent structure minus the susceptibility factor times the sum of all the Hammett constants sigma rho Due to promixity (steric) effects, influence of ortho substituents is hard to quantify. The same substituent in the ortho position may have a different effect on pKa for different acids. Hammett constants can be used to predict properties other than pKa Specifically, rate constants for hydrolysis (chapter 13) Also, redox potential? Chlorobenzenes: Hammett constants Cl (ortho) exptl 2.01 bond 1.23 comp 1.17 R2 0.63 0.90 0.99 Cl (meta) = 0.37 Cl (para) = 0.23 Exptl without trichlorobenzenes: Cl (ortho) = 1.53 R2 = 0.985 Taft correlation pK a pK E s * a CH 3 * Similar to Hammett correlation, but applicable to aliphatic systems. Reference compound has methyl group at the position of the substituent. Influence of substituent on pKa is divided into polar (electronic) and steric effects. * = polar substitutent constant * = susceptibility of backbone to polar effects Es = steric substituent constant = susceptibility of backbone to steric effects also used to predict reactivity . . . see Chapter 13 Partitioning Behavior of Organic Acids and Bases pH dependence of solubility: speciation HA H+ + A- HA (pure liquid or solid) Solubility is the equilibrium partitioning of a compound between the pure liquid phase and water. The solubility and activity coefficient of HA (the neutral form) depends on its size, polarity, and H-bonding ability. The intrinisic solubility of HA is not affected by acid-base reactions. However, the apparent solubility is highly dependent on pH due to protonation. the charged species has a much higher solubility than the neutral form. represents the fraction of the total amount of the compound that is in the neutral form. To determine the total solubility of an ionizable compound, first determine the solubility of the neutral form, then determine at the given pH. HA HA A 1 1 10 pH pK a sat C w , tot sat C w ,HA similarly, for B: sat C w ,tot sat C w ,B 1 Henry's Law (air-water partitioning): Assume that the ionized form cannot volatilize (no ionized gases allowed!) Only the neutral species is avialable for air/water exchange D aw H A , A K ' H KH RT For base: D aw B , B H 1 K ' H 1 KH RT Octanol-water partitioning: ionized form can partition into octanol by itself or as an ion pair observations suggest Kow (HA) 100 Kow (A-) so, by analogy to KH: K ow H A , A K ow B , B H K HA ow 1 K B ow Problem 8-1 Represent graphically the speciation of 4-methyl-2,5dinitrophenol and 3,4,5-trimethylaniline, and 3,4dihydroxybenzoic acid as a function of pH (2-12). Estimate (if necessary) the pKa values of the compounds. Problem 8.2 Represent graphically the approximate fraction of (a) total 2,3,4,6-tetrachlorophenol and (b) total aniline present in the water phase of a dense fog (air-water volume ratio ~105) as a function of pH (pH range 2 to 7) at 5 and 25C. Neglect adsorption to the surface of the fog droplet. Assume and DawHi value of about 70 kJ/mol for TCP and 50 kJ/mol for aniline. All other data can be found in Appendix C.