Biochemistry 304 2014 Student Edition Acids, Bases and pH
advertisement

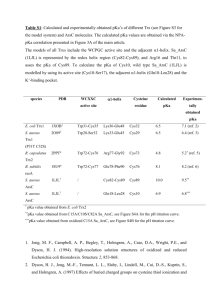
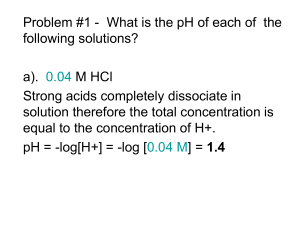
Acids, Bases and pH Student Edition 5/23/13 Version Dr. Brad Chazotte 213 Maddox Hall chazotte@campbell.edu Web Site: http://www.campbell.edu/faculty/chazotte Original material only ©2004-14 B. Chazotte Pharm. 304 Biochemistry Fall 2014 Goals •Review the ionization of water, Keq and Kw •Review the concept of an acid dissociation constant, Ka •Understand the concept of pH and a pH scale •Review the Henderson-Hasselbalch equation & its use •Be able to calculate the pH of a weak acid •Understand buffers •Review titration curves for monoprotic & polyprotic acids •Review the effect of pH on protein solubility and enzyme function •Review the concept of, and the calculation of, ionic strength Ionization of Water Water has a slight tendency to undergo a reversible ionization. H2O H+ + OHSince free protons do not exist in solution one writes: H2O H3O+ + OH(H3O+, a hydronium ion) Proton jumping: the proton can rapidly jump from one water molecule to the next in one of the fastest reactions in solutions. Keq and Kw A+BC+D Keq = [C] [D] [A][B] [] concentration approximates the activity coefficient FOR WATER: H2O H+ + OH- Keq = [H+] [OH-] [H2O] In pure water at 25 ºC [H2O] =55.5 M i.e., essentially constant compared to ions Therefore we can write : (55.5 M) (Keq) = [H+] [OH-] = Kw (ion product of water) Kw = 1 * 10-14 @ 25.0 ºC Table: The pH Scale As defined by Sorensen pH = -log [H+] pH + pOH = 14 pH is a shorthand way of designating the hydrogen ion activity of a solution Voet, Voet & Pratt 2013 Fig. 2-16 The ion product of water is the basis for the pH scale Lehninger 2001 Table 4.2 The “p” in pH designates the negative logarithm Sum of pH and pOH always =14 >pH 7 basic pH’s of Some Aqueous Fluids [H+] < [OH-] pH 7 neutral [H+] = [OH-] <pH 7 acidic [H+] > [OH-] Lehninger 2000, Figure 4.13 Acids & Bases - Definitions Brønsted and Lowry: Brønsted Acid - a substance that donates protons (hydrogen ions) Brønsted Base - a substance that accepts protons (hydrogen ions) When a Brønsted acid loses a proton a Brønsted base is produced. In this context the original acid loses a proton and produces a base, These are referred to as a conjugate pair, e.g. HA and A-. A strong acid or base is one that ionizes almost 100% in aqueous solution. Ka (Dissociation Constant) Ka numerically describes the strength of an acid. K = [H3O+] [A-] [HA] [H2O] since [water] = 55.5 M in dilute solution, [H2O] ≈ constant Ka = K [H2O] = [H+] [A-] [HA] Strong acids Ka >> 1 Weak acids Ka < 1 Henderson- Hasselbalch Equation Shows the relationship of a solution’s pH and the concentration of an acid and its conjugate base in solution. [H+] = Ka ([HA] / [A-]) (1) pH = - log Ka - log ([HA] / [A-]) since pH = -log [H+] (2) pH = pKa + log ([A-] / [HA]) since pK = -log K (3) HA = proton donor When [HA] = [A-] And pH = pKa A- = proton acceptor then log ([A-] / [HA]) = 0 ! But the H-H equation cannot be used for strong acid or base. Henderson-Hasselbalch Calculation Calculate the pKa of lactic acid given that the concentration of lactic acid is 0.010 M, the concentration of lactate is 0.087 M and the pH is 4.80. [lactate] pH = pKa + log [lactic acid] [lactate] pKa = pH - log [lactic acid] 0.087 M pKa = 4.80 - log 0.010 M = 4.80 - log 8.7 = 4.80 - 0.94 pKa =3.9 Extent of Ionization Relationship of pH and pKa Weak acids pH = pKa pH = pKa + 1 pH = pKa + 2 pH = pKa + 3 pH = pKa + 4 % Compound Ionized ~50% ~90% ~99% ~99.9% ~99.99% Weak Bases pH = pKa pH = pKa - 1 pH = pKa - 2 pH = pKa - 3 pH = pKa - 4 ~50% ~90% ~99% ~99.9% ~99.99% Cairns “Essentials of Pharmaceutical Chemistry”2008 p.22 pH of a Weak Acid Solution Calculation I Problem: A weak acid HA is 0.1% ionized (dissociated) in a 0.2 M solution. A)What is the equilibrium constant for the acid’s dissociation? B) What is the pH of the solution? pH of a Weak Acid Solution Calculation II “A” HA Start 0.2M Change -(0.1% of 0.2M) = -(2 x 10-4M) Equilibrium 0.2M -(2 x 10-4M) [HA] A- 0 0 +(2 x 10-4M) +(2 x 10-4M) (2 x 10-4M) (2 x 10-4M) (2 x 10-4M) x (2 x 10-4M) [H+] [A-] Ka = H+ (0.2 M - 2 x 10-4M) = 4 x 10-8 Ka = 1.998 x 10-1 = 2 x 10-7 Segal 1975 pH of a Weak Acid Solution Calculation III “B” pH = log (1 / [H+]) = log (1 / [2 x 10-4]) = log (5000) = 3.7 Acid-Base Neutralization Calculation Problem How many ml of 0.025 M H2SO4 are required to neutralize 525 ml of 0.06 M KOH? Setup: 1 # moles H+ (equivalents) req. = # moles OH- (equivalents) present 2 Liters x normality (N) = # equivalents 3 Litersacid x Nacid = litersbase x Nbase 1 H2SO4 = 0.025 M = 0.05 N (two hydrogens per mole sulfuric) Litersacid x 0.05 M = 0.525 litersbase x 0.06 M 0.63 litersacid = (0.525 liters x 0.06 M) / 0.05 M Acids, Bases, Salts & Solutions Types of salts and solutions formed when acid and base combine Strong acid + Strong base → Strong acid + Weak base → Weak acid + Strong base → Weak acid + Weak base → Example of strong acid and weak base from above → acidic salt This is why: NH4+ Cl- ↔ NH4+ ClNH4+ + H2O ↔ NH3 + H3O+ Neutral salt Acidic salt Basic salt Neutral salt HCl + NaOH → Na+Cl- + H2O HCl + NH3 → NH4+ ClCH3COOH+ NaOH → CH3COO- Na+ + H2O CH3COOH + NH4OH → NH4+ CH3COO- + H2O Some Examples of Drugs Formulated as Salts Diphenhydramine hydrochloride (Benadryl) Naproxen sodium (Aleve) Cetirizine hydrochloride (Zyrtec) Morphine sulfate Oxycodone Hydrochloride (Oxycontin) Cairns “Essentials of Pharmaceutical Chemistry”2008 p11 Buffers The maintenance of a relatively constant pH is critically important to most biological systems. pH changes can effect the structure, function, and/or reactivity of biomolecules. One pH unit is equivalent to a 10-fold change in H+ ion concentration. A buffer (system) resists changes in solution pH by changes in the concentration of the buffers’ acid and conjugate base (HA and A-). This can be illustrated in the titration curve of a weak acid. A system’s maximum buffering “capacity” is at or near the pKa of the molecule with a range typically ± 1 pH. Titration Curve Examples Acetic Acid, Phosphate & Ammonia CH3 HA H+ + AKa = [H+] [A-] [HA] % titrated COO- CH3COOH 0 50 100 Voet. Voet & Pratt, 2013 Fig 2.17 5.76 4.76 3.76 Titration of a Polyprotic Acid e.g., H3PO4 A polyprotic acid has a pKa for each ionization or ionizable group. The ionization of one group creates an electrostatic charge that raises the pKa of the subsequent ionization Voet. Voet & Pratt, 2013 Fig 2.18 A Buffer System: Acetic Acid / Acetate Adding H+ ions drives the equilibrium to acetic acid taking up the added H+ ions while adding OHions drive the equilibrium to acetate. Sum of the buffer components does not change, only their ratio. Lehninger 2000, Figure 4.17 The Bicarbonate Buffer System (Lungs & Blood) (Voet et al. Rx #2) (Voet et al. Rx #1) Lehninger 2000, page 105 pH and Solubility of a Protein At a protein’s isoelectric point, called pI, the sum of the positive charges equals that of the negative charges leaving no net charge At the pI a protein is least soluble. Matthews et al 1999 Figure 2.21 pH Optima of Three Enzymes Lehninger 2000, Figure 4.19 Ionic Strength A measure of the total concentration of ions in solution. The more charged the ion the more it is counted μ = ½ Σ ciZi 2 where ci = concentration of ith species and Zi = the charge on the ith species Why care? The greater the μ of a solution the higher the charge in the “ionic atmosphere” around an ion and the less net charge so the less attraction between any given cation and anion. Relates thermodynamic activity, ai, to concentration [A]. aA = [A] γA (see also extended Debye-Hückel Equation) Sample Calculation of Ionic Strength What is the ionic strength of a 0.015 M solution of CaCl2 ? The equation is = ½ ciZ i 2 The reaction is CaCl2 Ca2+ + 2 ClThe concentrations are [0.015] + [0.030] = ½ ((0.015 x 22) + (0.030 x -12)) = ½ ((0.060) + (0.030)) = ½ ((0.090)) = 0.045 The Effect of Ionic Strength Matthews et al 1999 Figure 2.22 End of Lecture